NNadir
NNadir's JournalWilliam Francis Gray Swann
I always do some light reading at bedtime, usually history, and the history book I'm reading now is this one: Big Science: Ernest Lawrence and the Invention that Launched the Military-Industrial Complex
Chapter 2 is a brief, pre-fame biography of E.O. Lawrence, his youth and college career. (His freshman year at a Lutheran University his mother insisted he attend, St. Olaf's, this future Nobel Physics Laureate who won for the invention of the cyclotron got a C in religion and a D in electricity and magnetism.) He dropped out of college for a while, then surreptitiously began to attend classes at the heathen University of South Dakota, where a physics professor took him under his wing, announcing to the classes that this undergraduate student is "going to be famous some day."
So he was.
After graduating from USD, Lawrence went on to graduate school studying under an iconoclast, William Francis Gray Swann at the University of Minnesota, then following him when he moved to Yale, where Lawrence was awarded his Ph.D. Intrigued by what I read in the brief pages describing him, I looked into Swann, whose patent unorthodoxy inspired Lawrence.
Here's a brief biography of his from the American Philosophical Society, which holds his papers:
Born at Ironbridge, England on August 29, 1884, Swann demonstrated an early aptitude for music, but little in the sciences. While his love for music continued to grow, however, Swann nevertheless elected to pursue what seemed to be a more practical course in medicine when he entered Brighton Technical College as a scholarship student in 1900. At Brighton, Swann was introduced to James Clerk Maxwell's treatise on electricity and magnetism, and swayed by its elegance and precision, he switched from medicine to physics, transferring to the Royal College of Science, from which he received his BSc in 1905.
As a Junior Demonstrator at the Royal College, Swann gained valuable teaching experience while strengthening his background in the "practical things of science" by studying electrical engineering. His combination of experimental and teaching prowess earned him an appointment as Assistant Lecturer and demonstrator at the University of Sheffield in 1907, where he worked while completing his doctoral studies at the University College London. He received his DSc in 1910. It was the first stop in an upward climb into increasingly prestigious academic appointments.
Despite low pay and less than ideal working conditions, Swann displayed sufficient promise in mathematical physics, electrodynamics, and in the new quantum theory that he garnered the attention of the Carnegie Institution of Washington. Lured away from Sheffield in 1913, Swann was appointed Chief of the Physical Division at the Department of Terrestrial Magnetism, spending much of the next four years in the design and production of an apparatus to assist in magnetic and atmospheric-electric observations aboard the ship Carnegie, but with his reputation rising, other offers were not long in coming.
After war-time service working on submarine detection with the National Bureau of Standards and with the army to determine why their balloons were prone to explosion, Swann accepted a standing offer to join the faculty at the University of Minnesota. An administrative prodigy, Swann developed the graduate program with such remarkable success, reaping the rewards along the way, that by the time he left in 1923, he had become the highest paid professor at the University. Two of his own students won National Research Council Fellowships, but in his own opinion, his greatest success was in mentoring a young Edwin O. Lawrence.
The next stop in Swann's academic climb was the University of Chicago, who in 1923 offered Swann a substantial increase in pay to replace Robert Millikan. Even before he accepted, however, Yale approached him with even more lucrative honor: full professorship, Director of the supremely well-equipped Sloane Laboratory, and responsibilities for only one postgraduate course. As Yale was courting, other offers poured in, including the Franklin Institute in Philadelphia, who struck up what Swann called a "fliratation" in connection with a proposed research institute for "practical Electrical Engineering." Funded by a bequest from Henry W. Bartol (d. 1918), a prominent industrialist and member of the Franklin Institute, the institute got off the ground in 1925, when Arthur Bramley became the first Bartol fellow even before a facility or staff were available.
Faced with a Hobson's choice of Chicago, Yale, or the prospective Bartol, Swann chose Yale. The Ryerson Lab at Chicago, he reasoned, was crowded and antiquated, and the research opportunities at Yale offered by Pres. James B. Angell were simply too attractive to turn down. After only one year at Chicago, and despite his department's pleas, Swann therefore moved to New Haven, bringing Lawrence in tow. As the Bartol got off the ground, however, Swann soon reconsidered, and when the number of fellows rose to five, he was tapped as the first Director of the Bartol Research Foundation.
One of Swann's first acts as Director was to secure an agreement with Swarthmore College to relocate the institute from its temporary quarters in Philadelphia to facilities on the college campus. Always meticulous and detail-oriented, his administrative oversight extended even to minor custodial expenditures, yet he was well regarded by the fellows and staff he oversaw, however his attentions appear to have been well appreciated by Bartol fellows. Swann was concerned that "the great industrial rese'rch laboratories" instilled a culture that led physicists away from "using their own hands," and he therefore insisted on manufacturing his own apparatus, including blowing his own glass, and he was adamant that his fellows follow suit. On a personal level, several fellows considered Swann to be something of a "father confessor" as well as scientist.
An immensely productive researcher, who wrote over 250 publications during his career, Swann continued to blend theoretical and empirical approaches, evolving as rapidly as his discipline to touch on relativity theory, condensed matter physics, atomic structure, matter, antimatter, and gravitation. He was best known, however, for his pioneering work on cosmic rays and high energy physics. In the late 1920s and early 1930s, he developed a mechanism for accelerating charged particles to cosmic ray energies by means of changing magnetic fields, a device he named the cygnatron ("swan tube" ). More in the public eye, he took part in organizing a number of high profile projects to investigate cosmic ray intensities at high altitudes, including a series of manned balloon flights funded by the National Geographic Society and the U.S. Army. Swann subsequently took these studies to airplanes, ships, underwater, and on mountain tops. Late in his career, Swann developed a keep interest in the relationship between religion and science and in psychic research.
As charismatic in presentation as he was dramatic, Swann was a natural teacher and effective public spokesman for science at various levels. Throughout his tenure at the Bartol, he conducted seminars for high school students at the Franklin Institute, he lectured on electrodynamics at the Moore School of Electrical Engineering at the University of Pennsylvania, and later in life, was Professor of Physics at Temple University. His 1934 book The Architecture of the Universe, its interpretation of the new, and often abstruse developments in modern physics, was a major success with the broader public, and as a result, Swann was regularly called upon to appear on the radio and interviewed in the newspaper. With a slightly eccentric image and trademark shock of long white hair, he was a natural as well for television, hosting a weekly program in Philadelphia popularizing science during the 1950s.
Despite the demands of a rigorous schedule of research, administration, and public appearances, Swann managed to preserve time and energy for his great passion, music. An accomplished cellist who had studied under Diran Alexanian, Swann maintained an active social and musical correspondence with musicians and kept an active hand in the music scene in Philadelphia. In addition to his performing as a soloist and with various orchestras, he helped found the Swarthmore Symphony Orchestra, worked as assistant conductor of the Main Line Orchestra and as director of the Philadelphia Academy of Music, and was a supporter and honorary fellow of Trinity College of Music, London. Music entered deeply into his private life as well: After his first wife, Frances Mabel Thompson died in 1954, he married Helene Diedrichs, a former child prodigy, pianist and Chair of the Piano Department at the Philadelphia Musical Academy. Mrs. Swann (who played professionally under her maiden name) had studied under Carl Wendling at the Leipzig Conservatory of Music and received degrees from the Royal Academy of Music and the Tobais Matthay Pianoforte School.
Swann was elected a member of the American Philosophical Society in 1926, and served as vice president of the American Association for the Advancement of Science (1923-1924) and president of the American Physical Society (1931-1933). He received honorary degrees from Yale (1924), Swarthmore (1929), Temple (1954), and was made a fellow of the Imperial College of Science in Technology (1956) and was awarded the Elliot Cresson Medal of the Franklin Institute (1960) for his research on cosmic rays. The final honor awarded Swann vaulted him literally to the heavens: In 1967, the International Astronomical Union named a lunar crater in Swann's honor.
In August 1959, already 75 years old, Swann retired to emeritus status at the Bartol Institute and was succeeded by Martin Pomerantz. Unburdened by administrative duties, he continued to conduct research almost until the day he died, January 29, 1962. He was survived by Helene Diedrichs, two sons, William F. Swann and Charles P. Swann, and a daughter Sylvia Swann Briggs. Both sons became prominent physical scientists, Charles at the Bartol.
William Francis Gray Swann Papers
Swarthmore housed the Bartol until the 1970's after which it declined to renew its lease, as it was not part of the University, something now viewed with some regret:
Known for its pioneering studies of cosmic rays and work in nuclear physics, Bartol hosted an international contingent of scientists and researchers. During World War II, Bartol's principal work involved the development of magnetron cathodes. William Elmore, a member of Swarthmore's physics department, conducted research at Bartol at that time. After the war, basic research in solid state and surface physics continued, along with the resumption of cosmic ray investigations led by Bartol director W.F.G. Swann...
...Yet Bartol never became an integral part of the College and, even among the College's science faculty, was known well by only a few. When Bartol's lease came up for review in the 1970s, the College ended its relationship with the foundation and took possession of its building. The inability of both institutions to develop closer collaborations is now viewed by many on both sides as a lost opportunity.
Renamed the Bartol Research Institute, it moved to its present location at the University of Delaware in 1977. The building it once occupied on campus was renamed Papazian Hall and for 40 years housed the College's philosophy and psychology departments. It was demolished in 2017 and is the future site of the Biology, Engineering, and Psychology building.
From the Bartol History page at UD:
The Bartol Research Institute
A Brief History
Mr. Henry W. Bartol, a member of The Franklin Institute, died on the 19th of December, 1918, leaving behind a will and codicil that provided for the establishment of the Bartol Research Institute. In that will Mr. Bartol designated as residuary legatee the Franklin Institute of the State of Pennsylvania. He stipulated: "All the rest, residue and remainder of my estate, except such as is situated in France, I give, devise, and bequeath to the Franklin Institute... to be applied to the establishment and maintenance of a department of practical Electrical Engineering..." The codicil subsequently changed this to "...the founding and maintenance of an institute... the preference however, being given to workers or those making researches into electrical science."
Mr. Bartol was a prominent Philadelphia industrialist. Although his gift in 1918 was sufficient to fund an institute of scientific study, the birth of the Bartol Research Institute (then called the Bartol Research Foundation) was slow and difficult. It was not until the end of 1925 that the first Bartol Fellow, Dr. Arthur Bramley, was appointed. The first publication of research supported by the Bartol Research Foundation, and performed by Dr. Bramley, appeared in the January, 1926 issue of the Journal of the Franklin Institute. This report discussed the multiplet structure in the Zeeman effect. The number of Bartol Fellows rose to five and on February 3, 1927, Dr. W. F. G. Swann was elected by the Board of Managers to be the first director of the Bartol Research Foundation.
W. F. G. Swann was appointed the Director of the Bartol Research Foundation at the age of 43. Born in England, he was educated at Brighton Technical College, the Royal College of Science, University College, Kings College and the City Guilds of London Institute. Dr. Swann came to this country in 1913 as head of the Physical Division of the Department of Terrestrial Magnetism at the Carnegie Institute in Washington. Later he was Professor of Physics at the University of Minnesota, the University of Chicago and Yale, where he became Director of the Sloane Laboratory. A man of many talents, Dr. Swann was an accomplished cellist, founder of the Swarthmore Symphony Orchestra, a former assistant conductor of the Main Line Orchestra and former director of the Philadelphia Academy of Music.
By the time of his appointment, Professor Swann had already distinguished himself as an excellent teacher, an outstanding researcher, and an emerging leader of the scientific community. Although Dr. Swann is perhaps best known for his experimental and theoretical efforts in the area of cosmic ray physics, his research interests touched on many other disciplines such as condensed matter physics, relativity, and charged particle acceleration. In the last seven years of his life he had 22 publications on such diverse subjects as atmospheric electricity, thermal conductivity of solids, the restricted theory of relativity, matter, antimatter and gravitation, and charged particle acceleration to cosmic ray energies. His grasp of electromagnetism was far reaching and entered into most of his research. In his capacity as a professor he is perhaps best known as the advisor of Dr. E. O. Lawrence who subsequently was awarded the Nobel Prize for developing the cyclotron. Lawrence followed Dr. Swann from Minnesota, to Chicago, and then to Yale where he received his Ph.D. Altogether Dr. Swann had over 250 publications including a well known book "The Architecture of the Universe". In 1967 the International Astronomical Union honored Professor Swann when it gave his name to a crater on the lunar surface at 52 º north latitude and 112 º east longitude.
Shortly after his appointment as Director of the Bartol Research Foundation, Dr. Swann secured an agreement with Swarthmore College to move the Foundation from its temporary lodgings in Philadelphia to the home campus of the college where it was able to enjoy the benefits of a college atmosphere. During the early 30's Dr. K. T. Bainbridge, then a Bartol scientist, developed a magnetic spectrograph with which he was able to make accurate mass determinations of low Z elements including 6Li using their accelerator. At about the same time Cockroft and Walton performed measurements on the 7Li + p = 2 reaction using their accelerator. Bainbridge was then able to verify Einstein's famous principle of mass- energy equivalence using the established masses for the proton and 7Li.
Several "high" altitude manned balloon flights were made in 1934 and '35 for the purpose of studying cosmic rays. Two of these, one of which crashed on descent, were sponsored by the National Geographic Society and the Army Air Corps and were flown by Air Corps personnel; fortunately the men on the crashed flight were able to eject and come down on parachute. Three other flights were flown by Dr. Jean Piccard and his wife. All of these flights contained a significant amount of Bartol equipment for the study of cosmic rays. Related investigations of cosmic rays were pursued from mountain tops, airplanes and ships, underwater, and in unmanned balloons.
Bartol became further involved in nuclear physics research with the construction of a 2.5 MV Van de Graaff accelerator under the guidance of Dr. W. E. Danforth. Bartol personnel also constructed a cyclotron in the late 1930's, the first cyclotron outside of Berkeley. This machine was actually built for The Franklin Institute's Biochemical Foundation, which was housed in the present Penny Hall of the University of Delaware. An extensive nuclear physics program did not develop until after World War II, with the completion of the 2.5 MV Van de Graaff and the construction of a second Van de Graaff with a potential of 5 MV. The principal research interests during the war, conducted in close collaboration with the Radiation Laboratory at the Massachusetts Institute of Technology, involved the development of magnetron cathodes. Basic research in solid state and surface physics continued after the war, in parallel with the resumption of cosmic ray investigations. Bartol's scope was further expanded in the 1960's with the initiation of research programs in astronomy and astrophysics...
Cool.
The Franklin Institute is still in Philadelphia, and when my kids were small, I was a member, and took them there for many hands on science demonstrations. (It's one of the reasons why raising children in New Jersey is a wonderful experience.) Mostly now, the institute is geared more for children than adults, but I think there is still some scientific research conducted there and some activities geared for adults.
I never knew however about the Bartol Institute, William Francis Gray Swann, and his role in the creation of E.O. Lawrence and the growth of "Big Science," which helped make America truly great, before philistines got a hold of it and began to dismantle the country to pay off debts to Putin.
I wish you a pleasant work week.
For the first time in this century, weekly CO2 readings at Mauna Loa are 25 ppm over 10 years ago.
Somewhat obsessively I keep a spreadsheet of the weekly data, which I use to do calculations to record the dying of our atmosphere, a triumph of fear, dogma and ignorance that did not have to be, but nonetheless is. I note, with sadness and regret, that we on the left are not free of such fear ignorance and dogma, although I wish we were. We cannot, with justice, attribute this outcome to Ronald Reagan, George Bush the first and second, and Donald Trump. We bear responsibility, no matter how much we pat ourselves on the back for our insane, and frankly, delusional worship of so called "renewable energy."
The amount of money "invested" in so called "renewable energy" in the period between 2004 and 2018 is over 3.036 trillion dollars; dominated by solar and wind which soaked up 2.774 trillion dollars.
Source: UNEP/Bloomberg Global Investment in Renewable Energy, 2019
This is an amount that is larger than the GDP of India, a nation with 1.4 billion people in it.
It is obvious that all the money thrown at so called "renewable energy" did not work, is not working. I, for one, am absolutely and irrevocably certain that even more money, tens of trillions of dollars, will not work.
The reason is physics. The laws of physics are not determined by popular opinion, delusional or otherwise. They are independent of politics, and politicians ignore them at the peril of all humanity. The reason that so called "renewable energy" has failed, is failing and will always fail is the low energy to mass ratio associated with it, along with its intrinsic variability.
For the first time since I've been keeping my spread sheet, the change in the concentrations of the dangerous fossil fuel waste carbon dioxide measured in comparisons of current weekly data is 25.00 ppm higher than that obtained 10 years ago.
Here is the data from the Mauna Loa observatory for the week beginning February 23, 2020:
Up-to-date weekly average CO2 at Mauna Loa
Week beginning on February 23, 2020: 413.72 ppm
Weekly value from 1 year ago: 412.25 ppm
Weekly value from 10 years ago: 388.72 ppm
Last updated: March 1, 2020
There is considerable noise in these measurements. The weekly comparisons with one year ago, going back to the first week of December, range from 1.47 ppm over the same week of this year as compared to the previous year (the week beginning on February 23, 2020) to 3.54 ppm (the week beginning December 29, 2019). These values are somewhat important since they are utilized to determine than annual increases.
The website has been updating the 2019 annual figure as we've moved through February: The current value is 2.46 ppm over 2019, making it the 8th highest such measurement in the last 60 years of recording data.
There is another way of looking at annual increases (see below), but here is how the Mauna Loa observatory calculates annual increases:
The estimated uncertainty in the Mauna Loa annual mean growth rate is 0.11 ppm/yr. This estimate is based on the standard deviation of the differences between monthly mean values measured independently by the Scripps Institution of Oceanography and by NOAA/ESRL. The annual growth rate measured at Mauna is not the same as the global growth rate, but it is quite similar. One standard deviation of the annual differences MLO minus global is 0.26 ppm/year.
Using this method of calculation, there were, in the 40 year period between 1959 and 1999 five years in which the carbon dioxide concentrations of the dangerous fossil fuel waste carbon dioxide increased by more than 2.0 ppm.
In the 19 year period between 2000 and 2019 there have been 12 such measurements.
However there is another way to calculate annual increases in carbon dioxide from year to year, and one can do this using data on the website.
On the data pages of the Mauna Loa Observatory there is a link to Mauna Loa CO2 annual mean data which calls up a text file. If one utilizes imports this text file into a spreadsheet and calculates with it the data for 2019 calculates that the difference in the mean for 2019 with respect to 2018 was 2.92 ppm making it the third worst year after 2016 (3.43 ppm) and 1998 (2.97), the latter year reflecting the Indonesian/Malaysian forest fires when vast stretches of the rain forests burned as a result of the fires set to clear those forests for palm oil plantations went out of control. Palm oil became a hot commodity when Germany announced its "renewable energy portfolio standards" for biodiesel fuel.
If any of this troubles you, don't worry; be happy.
The old white racist guy in the White House says that climate change is a "Chinese plot," and the old white guy who leading candidate for the nomination in our party - even if he isn't in our party - says that all the world's energy problems can be solved with so called "renewable energy," but that more important than shutting down the dangerous fossil fuel plants is shutting the world's nuclear plants.
We on the left are not innocent.
Nuclear energy was the last, best hope of the human race for addressing climate change, but don't worry, be happy. After all, FUKUSHIMA!!!!!!
It may be the case that someone will die from radiation at Fukushima, and clearly that is more important than the 20,000 people who died from seawater in the same event because coastal cities have become dangerous, and it is definitely more important than the six to seven million people who will die this year, around 19,000 today, who will die from air pollution because people prattle on about how "nuclear power is too dangerous."
History will not forgive us, nor should it.
I hope you're having a pleasant weekend. Enjoy your Sunday evening.
You know...
...the only people here who want my resume all the time, seem to be Sanders supporting kids with pretty strong questions about my resume.
Is there any real reason that I have to educate Sanders supporters?
I recently had to put on my ignore list a really, really, really dumb guy who considered his Ph.D. in protein dynamics an exercise in - his words, not mine - "dick waving" while making idiotic comments on my intellectual credentials.
Apparently, this moron thought that because he was a "scientist," he knew all about climate change, even though he had clearly never opened a book on the subject in his narrow life.
I know the type very well.
If your claim is that because one hundred black intellectuals - who you do not list - support Sanders that therefore all black intellectuals support this tired dogmatic old white guy who hasn't changed his mind about a single thing since 1969, then clearly that says something about you and not black intellectuals.
How is it that I am required to list black intellectuals for your amusement? Who are you to me?
I get very, very, very tired of bad thinking, but let me try to address this....
There is a commonly understood practice in bad thinking called, Appeal to Authority.
A few years back, while wandering in the bowels of Princeton University's Firestone library I came across a strange little monograph in German written by a Nobel Laureate, Johaness Stark, the physicist who discovered the "Stark effect," the discovery that spectral lines were effected by magnetic fields. The effect is quite real, and remains, over a century after its discovery, an important physical fact.
The library has apparently moved the book off the shelves to "RECAP" a warehouse for books not commonly read anymore, but one can still get it if one is interested.
Here it is: Adolf Hitlers ziele und persönlichkeit, von dr. Johannes Stark
I think the University should have left it on the shelves as a lesson in appeal to authority arguments.
The book, written in 1932, before Adolf Hitler took power, which translates into "Adolf Hitler's Goals and Personality" is all about how wonderful Adolf Hitler would be for "German Science."
For further amusement, one can read the 1945 Farm Hall Transcripts where one German Nobel Laureate, Otto Hahn, tells another German Nobel Laureate, Werner Heisenberg, while they are discussing the subject of the practical results of American scientists, that he is a "second rater" whereupon Heisenberg agrees.
This may be found in the transcripts (translated into English with a foreward by Jeremy Bernstein) on page 118:
hahn: At any rate, Heisenberg, you’re just second raters and you might as well pack up.
heisenberg: I quite agree.
hahn: They are 50 years further advanced than we.
heisenberg: I don’t believe a word of the whole thing They must have spent the whole of their £500,000,000 in separating isotopes; and then it is possible.11
Hitler’s Uranium Club
The point is that it is possible to be an intellectual in one area, and be absurdly naive and even something of an idiot in another.
Here is my opinion: Senator Sanders is no intellectual, given the fact that his rhetoric is quite as simplistic as that of the orange racist in the White House. The fact that he is on the left and that he is not a racist, at least not overtly, makes him preferable, to be sure, to the orange idiot, but it is clear to me that as opposed to other Democratic candidates, he will be unable to arrest the destruction of our country.
If we wanted a left leaning President who is also a flexible thinker, we would vote for Ms. Warren, since she is intellectually and ethically very much more sophisticated than the tired old white Senator from Vermont. The tired old white Senator from Vermont rather like the MAGAT king, feels himself to be superior to all others, including the entire Democratic Party, purely an avatar of the Dunning-Kruger effect.
Now. Again. This is my opinion. It doesn't matter if I unload trucks at the West Windsor Walmart or his I am the Director of the Institute of Advanced Study, if I trim the bushes at Princeton University or if I am a tenured professor in the Materials Science and Engineering Department.
I can respect the thinking of powerful minds in one area, while completely discounting the thoughts of the same minds in other areas.
If there are 100 black intellectuals who support Senator Sanders, that's fine for them. This has nothing to do with whether Sanders would be as good a President as any of our other candidates, all of whom are quite happy to be Democrats and do not regard themselves as above the Democratic Party.
I would think we've had enough of Dunning Kruger avatars who hold themselves in high esteem, and I have no respect at all for the political opinions of any intellectual who cannot grasp this fact, no matter which giant ego they support, and no matter what their accomplishments might be. If they support Sanders, in my opinion, thinking for myself, they are fools.
Got it?
No?
Why am I not surprised?
Freeman Dyson has died.
Physicist/Mathematician/Iconoclastic thinker, Freeman Dyson, of the Institute of Advanced Study in Princeton has died.
His greatest accomplishment as a physicist was probably showing that three mathematical formulations of Quantum Electrodynamics, QED, were equivalent mathematically, those of Richard Feynman, Julian Schwinger, and Sin-Itiro Tomonaga. All three of the latter shared the Nobel Prize in Physics in 1965. Dyson did not win the Nobel Prize despite his contribution, something that Nobel Laureate Steven Weinberg described as Dyson having been "fleeced."
His obituary is here: Freeman Dyson, Math Genius Turned Visionary Technologist, Dies at 96.
Dyson knew all the greats of his generation: He was recruited to The Institute of Advanced Study by none other than the director there, Robert Oppenheimer, and worked and knew men like Hans Bethe.
He was a deep thinker, one of the first people along with Alvin Weinberg, to revive the idea first advanced by Arrhenius in 1896 that climate change would result from carbon dioxide accumulations. Ironically, in later years, he became something of an iconoclastic a climate skeptic, arguing not so much about the basic physical nature of the problem, but about the predictive ability of computational approaches to predicting its effects.
His obituary for Hans Bethe is here: Hans Bethe.
Here is an excerpt from that Obituary that Dyson wrote in Science, Dyson, Science 08 Apr 2005: Vol. 308, Issue 5719, pp. 219
SIGN UP FOR THE SCIENCE eTOC
Get the latest issue of Science delivered right to you!
Email address*
He continued to pour out a stream of research papers while carrying a full load of teaching and administrative duties and supervising an army of graduate students. When I was one of his graduate students, he came every day to eat lunch with us at the student cafeteria, sharing our problems and telling stories of his adventures in Germany and in Los Alamos. We learned even more at the lunches than we did at his lectures. Everyone called him Hans. He told us that one of the best things about moving from Germany to America was that nobody in America called him “Herr Professor.”
Bethe remained active as a physicist, doing calculations and publishing papers, well into his nineties. From the age of 70 to the age of 95, he enjoyed a fruitful collaboration with Gerald Brown, working out theories of supernova explosions and gamma-ray bursts. Brown has published a delightful account of the collaboration, with the title “Fly with Eagles” (2). Brown says, “I had to wait until he was more than 70 years old in order to have any chance of keeping up with him. He worked like a bull-dozer, heading directly for the light at the end of the tunnel.” The last time I talked with Bethe, he said, “It is a shame. Now I am 98 and I cannot be as active as I was when I was 90.”
Bethe carried in his head all the numbers that play an important role in physics or in engineering. Given any question, he could estimate a numerical answer with lightning speed. His estimates were amazingly accurate. He put this skill to good use when he helped Robert Wilson to design a succession of particle accelerators at Cornell. The same skill made him an ideal leader for the theoretical division at Los Alamos, designing the first atomic bomb during World War II and helping to design the first hydrogen bomb in 1952.
For the 60 years that he lived after 1945, he worked hard to educate the public about the facts of nuclear weaponry and the impossibility of winning a nuclear war. He was actively engaged in fighting for arms-control treaties and against escalations of the arms race. At the age of 90, he wrote in a letter to President Clinton: “The time has come for our nation to declare that it is not working, in any way, to develop further weapons of mass destruction of any kind. …You might consider making a suitable pronouncement along these lines, to discipline the bureaucracy, and to reassure the world that America is vigilant in its desire to ensure that new kinds of nuclear weapons are not created.” Now that he is dead, it is up to us to continue the good fight that he fought for nuclear sanity, moderation, and common sense.
(Although Dyson was Bethe's graduate student, he never actually completed his formal Ph.D. degree, but was recruited by Oppenheimer none the less.)
Dyson was a brilliant writer. I know this because I spent about two weeks reading through many of his delightful books, in preparation for an appointment I had to meet him in his office at the Institute for Advanced Study. A friend was able to get an appointment after I expressed disappointment at having missed one of his lectures because of a conflict with some unimportant business meeting that I was trapped into attending. My friend described some of my scientific work - my friend was not a scientist and kind of butchered what I actually did - and to my surprise Dyson emailed me to come to his office for a chat, "tea and cookies..." he wrote. I asked if I could bring my kids and he wrote back with some bemusement, saying "the more the merrier." I brought my sons and my youngest son's best friend - who would later be valedictorian of my son's high school class - and we spent a long and memorable afternoon.
As a person interested in nuclear engineering, I asked a number of questions about Alvin Weinberg and about his work on the TRIGA research reactor, which he designed in 1956 at the request of Edwin Teller to be a "melt down proof" reactor. When I asked him about it he was deferential, claiming that the reactor was really the work of Iranian metallurgist and UC San Diego Professor, M. Simnad, who was the designer of the uranium-zirconium-hydride fuel. TRIGA reactors still operate all over the world, serving the research community and preparing radioisotopes for use in medical procedures.
Later the conversation turned, strictly out of my curiosity to topics in nuclear weapons design, whereupon he politely stopped me saying, "I'm sorry but that's classified." The conversation was quite freewheeling, with me stopping to tell the kids why the topic was important - my son complains that he wishes he had been older so he could have appreciated the richness of the conversation - and I found that whatever topic I brought up, he could speak on it with authority and expertise. The conversation went so far as to discuss Stuart Kaufman's work on self ordering systems and his work exploring them as a feature in the origin of life, and I was startled when Dyson launched into an elegant discussion of the topic, punctuating it with sympathetic comments on a tragedy in Kaufman's personal life.
He was very pleased when I brought up the topic of his work on showing the constancy of fine structure constant over a long periods of time, something he was able to prove by examining the properties of certain samarium isotopes. He told us "that was the last piece of real science I ever did."
I could have stayed there for a year without ever losing fascination, but eventually the sun was going down, and as he was in his late eighties or early nineties at the time, it occurred to me that I was overstaying my welcome, although he did nothing to push me out.
I said that perhaps I should go, and he didn't argue, and turned on the light. We took some pictures, my son's friend of Dyson, my sons and me, me of him, my sons, and my son's friend. I just pulled them up to look at them. I certainly have a shit-eating grin in those that I'm in. I'm a short fat guy, and what's amazing is that I seem to tower of him; he was a giant in a small body.
There was a huge pile of books on the floor of the office, and as we were leaving, he invited each of us to take one. When we started to demure he insisted, since he said that the books on the floor were all sent to him my authors who asked that he read them; and he indicated he would never find the time to do so, and so he gave him away to visitors to his office. One of them was a book written by a friend of mine on the subject of thorium reactors. (I actually forget what book I took; it's around here somewhere.)
One of the interesting things was that he was not above "crazy" ideas. One idea was to dismantle the planet Jupiter in order to construct a device to capture all of the sun's energy.
That was, for me, one of the most memorable afternoons of my life; I will always treasure the pictures and the memory.
The world has just lost some fascination. He was, I've read, an iconoclastically religious man: "May flights of angels, carry you to your rest."
Demographics and Climate Impacts on Embodied and Materially Retained Household Carbon
The paper I'll discuss briefly in this post is this one: Clarifying Demographic Impacts on Embodied and Materially Retained Carbon toward Climate Change Mitigation (Shigetomi, et al., Environ. Sci. Technol. 2019, 53, 24, 14123-14133).
This paper is part of the ACS Author Choice system, and thus is open sourced. Anyone can read it, and therefore there is no need for me to excerpt it extensively or reproduce graphics as I do in most of my other posts.
The point of the paper is to discuss embodied carbon in households in developed nations, in this case, Japan.
In various places, here and elsewhere around the internet, I have argued that we are well beyond the carrying capacity of the planet as a whole. In general, in most biological systems, populations can, and do, exceed the levels that can sustain such a population. In fact, an ecosystem can produce so much waste as a result of an overextended population as to eliminate the species in a particular closed system. This is most commonly observed in fermentation. The accumulation of alcohol, the waste product of yeast metabolism, in a wine cask or in a fermentation tank digesting grain ultimately kills all the yeast, and the population collapses.
It is the case of the nominally cognizant biological species, that is human beings, us, that we are not all that much different than yeasts. Yeasts don't know anything at all about population dynamics and do not know or care that they are biological species, whereas some human beings might be able to intellectually recognize that they are biological species as well - although many assume that we are somehow special, "higher" forms of life - but otherwise behave rather like yeasts. As a species, we are choking on wastes.
It seems fairly obvious to me and many other people that the planet's population is not sustainable, but as a cognizant species, it is conceivable, but not apparently practical, that population reduction might take place outside of a yeast like catastrophe. Elsewhere I wrote:
Current Energy Demand; Ethical Energy Demand; Depleted Uranium and the Centuries to Come
Japan is a country with a declining birth rate; if I recall correctly the replacement rate is lower than the death rate and therefore the population of native Japanese is falling, not rising. Of course, Japan is a first world country, and the per capita consumption rate is relatively high - not as high as the per capita consumption rate of Americans - but higher than that of most citizens of this planet.
When I was born, the concentration of the dangerous fossil fuel waste carbon dioxide in the planetary atmosphere was probably less than 310 ppm. The rate of increase was apparently less than 1.0 ppm per year. As measured last week at the Mauna Loa observatory, it was over 414 ppm. The rate of increase over the last decades is roughly 2.4 ppm per year.
My generation consumed most of the world's resources, is still consuming most of the world's resources, and will continue to consume until we die. We are killing the future of our species, and apparently couldn't care less.
History will not forgive us, nor should it.
The interesting point about this paper is that we won't be done destroying the environment when we die off leaving all future generations to deal with the consequences of our stupidity: The embodied carbon we leave behind will be damaging the environment when we have either rotted or been incinerated.
From the introductory text to the paper:
The carbon physically contained in products at the global and local scale has been analyzed in previous studies.(10?14) Lauk et al.(11) found wood products and plastic, respectively, accounted for 61% and 17% of total carbon stored in socioeconomic stocks around the globe in 2008, highlighting that per-capita plastic usage has shown high growth since 1900. Further, Ohno et al.(5) first addressed the linkage between the amount of carbon contained in products and final consumption. This research elucidated the structure of physical carbon retention for both wooden and petroleum-derived products, namely, materially retained carbon (MRC) induced by Japanese final consumption in 2011, finding that 14.2 Mt of carbon was newly added in Japan, corresponding to 4.1% of total annual CO2 emissions in the same year if it were all incinerated. Passenger motor cars are the largest contributor, followed by plastic products, cosmetic products, and apparel goods...
The authors argue for a closed carbon loop as a policy initiative, particularly with respect to the carbon reserve represented by polymers. Of course, people have been arguing this with increasing vehemence for most of my lifetime; the reality is that these are not working, and in any case, the driver of a closed carbon loop cycle is energy. Unless you have energy, this cannot be done.
I have also argued, on thermodynamic grounds, that the ultimate and highest efficiency forms of energy are found in high temperature reforming of carbon based products. If anyone thinks that this type of energy will come from solar or wind energy, or even more dangerous fossil fuel use - the use of fossil fuels is increasing rapidly, not declining - they are lying to themselves.
I think the article is an interesting read. It's not overly technical, not as technical as many of the other papers I discuss in this forum.
If interested, check it out. It's free, a gift from the American Chemical Society to public discourse.
Have a happy Friday.
Trends in Nitrate, Arsenic, and Uranium in Groundwater Beneath Irrigated Cropland.
The paper I'll discuss in this post is this one: Using Age Tracers and Decadal Sampling to Discern Trends in Nitrate, Arsenic, and Uranium in Groundwater Beneath Irrigated Cropland (Anthony J. Tesoriero, Karen R. Burow, Lonna M. Frans, Jonathan V. Haynes, Christopher M. Hobza, Bruce D. Lindsey, and John E. Solder, Environmental Science & Technology 2019 53 (24), 14152-14164)
In recent years, I have paid some attention to the destruction of the Ganges River in India - the sacred Ganges among some of the faithful - particularly as it effects the delta nation of Bangladesh. The lower flows have resulted in increased reliance on groundwater for irrigation in Bangladesh, with the concomitant result, since Bangladesh sits on arsenic rich rock formations, with mass poisoning of the population there. One can read about this - among many other places - in another paper in the same journal that features the paper I will discuss: Effectiveness of Different Approaches to Arsenic Mitigation over 18 Years in Araihazar, Bangladesh: Implications for National Policy (Nadia B. Jamil, Huan Feng, Kazi Matin Ahmed, Imtiaz Choudhury, Prabhat Barnwal, and Alexander van Geen, Environmental Science & Technology 2019 53 (10), 5596-5604)
This latter paper contains the following text:
Progress. "Only" 40 million. We need some "renewables will save us" types to come over and make us feel better with percent talk. You know, "Arsenic poisoning in Bangladesh has decreased by 30%!!!!!"
My interest in the Ganges was motivated by that river's role in uranium transport in the Earth's geochemical uranium cycle, when I was doing background research for a post elsewhere on the internet: Is Uranium Exhaustible.
The Ganges transports about 1200 tons of uranium per year to the ocean: Krishnaswami and J. Kirk Cochrane, eds. U-Th Nuclides in Aquatic Systems. Chapter 10, J. Kirk Cochrane and David Kadko, page 293. See also Dunk, R. M., R. A. MiUs, and W. J. Jenkins. Chemical Geology 190, 45-67 (2002)
The transport of naturally occurring elements from the ores in which they are found are obviously affected by environmental chemistry and physical processes. This should be obvious since we use chemistry to isolate elements from their ores. A widely used reagent for the recovery of uranium from its ores - one such ore is used nuclear fuel - is nitric acid, HNO3. Salts of nitric acid, in particular the ammonium salt - an explosive salt that was utilized by the right wing terrorist Timothy McVeigh to blow up a building in Oklahoma City, who may be awarded posthumously the Presidential Medal of Freedom by the anti-Freedom Nazi in the White House (would you really be surprised) - are widely used in agriculture.
It follows that nitrate can mobilize uranium in rocks, and the physical process of pumping water over them, can further exacerbate this extraction process.
From the text of the paper under discussion:
High concentrations of arsenic and uranium occur in groundwater in irrigated areas due to desorption, redox conditions, and evaporative concentration.(4)Irrigation-induced increases in microbial activity and water–rock interactions or the land application of lime often lead to increases in bicarbonate and calcium in groundwater. These increases in bicarbonate and calcium favor the formation of Ca–U(VI)–CO3 complexes that increase the desorption of U from subsurface sediments(5,6) and/or the dissolution of uranium-bearing minerals,(7) leading to higher uranium mobility. The effect of bicarbonate and calcium on uranium mobility is evidenced by the strong correlation between bicarbonate and uranium concentrations in groundwater in a large regional study in Germany(8) and by concordant changes in bicarbonate and uranium concentrations in a national study in the United States.(9)
Infiltrating irrigation water may also alter redox conditions which may affect the mobility and toxicity of contaminants either by affecting the transformation of contaminants to other species (e.g., creating more oxic conditions which may limit denitrification,(10) arsenic reduction(11)), or by causing the precipitation or dissolution of compounds that contain or sorb contaminants (e.g., sorption of arsenic on iron oxides(12)). Irrigating cropland introduces electron acceptors such as dissolved oxygen and nitrate, which can cause the oxidative dissolution of reduced compounds containing constituents of concern (e.g., arsenic, uranium). The correlation of nitrate concentrations in groundwater with selenium and uranium has been suggested as evidence that nitrate may serve as an electron acceptor in the dissolution of selenium and uranium-bearing minerals.(13,14) In one large regional study, the highest concentrations of uranium were observed in manganese and nitrate-reducing conditions,(8) while laboratory studies have found that nitrate is a stronger oxidant of uranium than dissolved oxygen.(15)Arsenic concentrations in groundwater are also often affected by redox conditions due to the release of sorbed arsenic during the reductive dissolution of iron hydroxides.(12,16) While high arsenic concentrations are often associated with reducing conditions, the oxidative dissolution of arsenic-bearing sulfides may also result in elevated arsenic concentration in certain environments.(17,18)
The authors discuss two areas in which the concentration of these two elements and the nitrate ion have been tracked.
The authors chose two relatively arid regions where there is intensive irrigated agriculture:
... Groundwater in the basalt units flows preferentially through the tops and bottoms of individual lava flows, rather than through the less permeable interior.(24)
Figure 1 in the paper is a map of this region.

The caption:
I once encountered a really dumb guy or gal on this website who proudly announced to me that nuclear power is "too dangerous" because a tunnel containing some old chemical reactors at the Hanford Nuclear Weapons plant collapsed. This was in lieu of giving a shit about the 19,000 people who would die each day from the time of his post up to and including today from air pollution. That moron of course, has made it to my wonderful ignore list, but it is notable that the Hanford reservation contains tanks that are rich in nitrate and nitrite and uranium.
You hear these sort of things, but you really don't want to believe that people say these sort of things.
The authors of this paper claim that although the Hanford tanks are well known to contain huge amounts of nitrate and uranium this has had little bearing on their findings:
Reference 27 is here: Water Quality of the Lower Columbia River Basin: Analysis of Current and Historical Water-Quality Data through 1994
Reference 28 is here: USGS Water Data for the Nation
Poking around in reference 28, one can learn of the concentrations of uranium in groundwater in Washington State well upstream from the Hanford reservation. Concentrations of Groundwater Uranium Upstream in the Columbia River Watershed, part of this report: Uranium concentrations in groundwater, northeastern Washington Although the authors of this report and the authors of the paper under discussion all work for the USGS, the data does seem to suggest that uranium concentrations in ground water in Washington State are not a function of the existence of the Hanford tanks, although credulous anti-nukes will not believe it.
In any case, the authors second study area is in Nebraska, also in an irrigated region:
This study area has a humid, continental climate,(20) receiving an average of 68 cm of precipitation each year.(31) Cropland, primarily corn and soybeans, is prevalent in the study area, with most of this cropland receiving irrigation. Rates of recharge from precipitation are estimated to be 14.2 cm/yr, with irrigation recharge estimated to be 6.4 cm/yr.(31) While groundwater has been used for irrigation in the High Plains area since the late 1800s, intensive irrigation did not occur until the mid-1900s. Irrigated cropland began to increase dramatically in the 1950s in the Nebraska portion of the High Plains aquifer; (32) climate change may result in future increases in irrigation demand in this area.(33)
Fertilizer applications have increased markedly since 1950, resulting in dramatic increases in nitrate concentrations in recharging groundwater in recent decades.(3,34) Stanton et al. (2006) sampled shallow groundwater from 30 wells in the High Plains aquifer in 2004 and determined that nitrate concentrations in shallow groundwater ranged from 2.0 to 106 mg/L as N, with a median concentration of 10.6 mg/L.(29) Nitrate-N isotope ratios in agricultural recharge suggest that fertilizer is the primary source of nitrate in groundwater recharge in this area.(3)
The study area:

The caption:
Figure 2. Site map of High Plains aquifer wells sampled for this study. Map is from Arnold et al. (2018, https://pubs.er.usgs.gov/publication/ds1087). Identification numbers correspond to hpgwvfps1 listings in Table 1 of Arnold et al. (2018), where water quality and construction details are provided for each well.
The age of the water tested is determined by the concentrations of the radioactive hydrogen isotope tritium injected into the planetary atmosphere by nuclear weapons testing, particularly by hydrogen bombs:
Groundwater samples were analyzed for tritium at all sites and sulfur hexafluoride (SF6) at the High Plains sites. Samples were characterized as premodern, mixed, or modern using the tritium category method.(46) A brief discussion of the tritium category method is provided below, with details provided elsewhere.(46) The tritium category method relies on variations in concentrations of 3H in groundwater given the temporal and spatial variation of 3H in precipitation.(47)3H concentrations in precipitation were at low, naturally occurring levels before 1953 but subsequently increased rapidly due to above-ground nuclear bomb testing (Figure 2b in Lindsey et al., 2019).(46)3H concentrations in precipitation remained high for many years, only returning to near prebomb concentrations in the past decade. However, 3H concentrations in groundwater that recharged prior to 1953 are much lower than recharged in the postpeak era due to the decay of 3H between 1953 and the sample collection date. This decayed concentration of recharge just prior to 1953 represents the upper threshold concentration of what is classified as premodern water. Conversely, a 3H concentration in groundwater that is greater than the lowest decayed concentration expected from precipitation during the postpeak period must have recharged after 1952 and is classified as modern water. Lastly, 3H concentrations that are between the upper and lower threshold are the result of a mixture of modern and premodern water and are termed mixed water. Groundwater ages of modern samples at the High Plains site were further refined by measuring SF6 concentrations in groundwater samples and relating these concentrations to atmospheric inputs.(48)
SF6 is a persistent greenhouse gas, with a global warming potential of 23,900 relative to CO2. It is totally anthropogenic, and has been industrially synthesized to replace PCB's in electrical transformers and to make those wonderful insulated solar windows in modern McMansions. Thus its presence in water is a time marker, given that the gas has only existed in prominent concentrations in recent times; it does not occur naturally.
Here are tables of results from the paper:

Some other graphics:

The caption:

The caption:

The caption:

The caption:
Figure 6. Boxplots of nitrate concentrations in modern groundwater as a function of irrigation water source at the Columbia Plateau site. Samples collected in 2014. Number above each plot indicates number of samples used in calculation. Some outliers are not shown.

The caption:

The caption:
According to the authors, it does appear that while the concentrations of these analytes has increased since the beginning of irrigation, but the concentrations have stabilized since the early 2000's.
There is some discussion in the paper of the role of phosphate, which along with nitrate, is an element of commercial fertilizers on which the world food supply depends:
Although phosphate here is discussed in connection with its cogener anion arsenate, it is well known that uranium is frequently a constituent of phosphate minerals and historically, before minerals were discovered having a higher concentration, phosphate mines were often considered as uranium ores in addition to phosphate ores. As a result of not removing uranium from phosphates, uranium is widely distributed on agricultural fields as a constituent of fertilizers.
A recent publication, albeit from a few years back, has examined the question of whether uranium should be mined from phosphate: To Extract, or not to Extract Uranium from Phosphate Rock, that is the Question (Haneklaus et al., Environ. Sci. Technol. 2017, 51, 2, 753-754)
It's open sourced; anyone can read it. It contains this text, which is not hostile to the only technology with even a remote chance of addressing climate change, nuclear energy:
"ISL" is a technology known as "in situ leaching."
I have argued in many places, here and elsewhere, that uranium mining need not be necessary at all for centuries. If we simply convert the uranium already isolated into plutonium, all of the world's energy needs for all purposes, can be met using uranium (and waste thorium from lanthanide mine tailings) already mined. However it is also possible to recover uranium in remediation schemes for groundwater with mobilized uranium as a result of nitrate leaching, such as that resulting from irrigation or - in the case of abandoned "fracking" fields like those in California and Pennsylvania, from abandoned oil and gas wells. (Coal ash is also a potential source of uranium.) Recovery of this type of uranium as a side product of remediation of natural uranium mobilization as a result of agricultural or industrial processes may well extend the time that the use of uranium can prevent the need for any energy mining, including the disastrous mining of oil, gas and coal, and for that matter, steel, copper, lanthanides and bauxite to support the pixilated so called "renewable energy" industry.
Nuclear technology has the capability of offering sustained high temperatures, and thus has the possibility of running at much higher thermodynamic efficiency than any other technology, meaning that even at current levels of energy production, running about 600 exajoules per year, the benefits of energy might be extended more broadly to those who lack it. (On the other hand, Jevon's paradox does offer a warning counter intuitive caveat on the benefits of efficiency.)
To return to the paper cited at the outset, here is the authors' concluding summary of the paper under discussion:
These findings suggest several areas that should be prioritized when monitoring groundwater beneath irrigated cropland. First, high nitrate concentrations in shallow modern groundwater have been sustained for decades and pose a future risk to deeper groundwater used for drinking water. Given the oxic conditions of these aquifers, nitrate concentrations may be expected to increase in older modern water as nitrate in the shallow portion of the system continues to migrate into deeper portions of these aquifers. Second, increased monitoring for trace metals in shallow groundwater beneath irrigated areas is warranted, as these contaminants may be mobilized by changes in water chemistry: increases in bicarbonate may mobilize uranium and increases in phosphorus may mobilize arsenic. Third, areas that use groundwater for irrigation may have an elevated risk of high nitrate concentrations due to the repeated dissolution of land applied fertilizers during recirculation.
I trust you are enjoying your weekend.
I'm very concerned that my cat will vote for Trump.
She has a brain the size of a walnut.
Material balance evaluation of pyroprocessing for minor actinide transmutation nitride fuel.
The paper I will discuss very briefly is this one: Material balance evaluation of pyroprocessing for minor actinide transmutation nitride fuel. (Sato et al., Journal of Nuclear Science and Technology. 2020, VOL. 57, NO. 3, 224–235.)
The actinide nitrides are very attractive nuclear fuels because of their high thermal conductivity and the ease by which they are reprocessed. During his quest to learn how to fix nitrogen, Fritz Haber explored a uranium catalyst for the purpose. Uranium nitride when exposed to water rapidly decomposes to give aqueous ammonia and uranium oxides. (Haber was able ultimately to utilize hydrogen and ammonia to accomplish the task, which was then industrialized by the German chemical engineer Carl Bosch. The invention allowed Germany to sustain itself for four years in the first world war without access to Chilean salt peter.)
The property makes it very convenient to reprocess nuclear fuels.
The paper above is about an ADS system, an accelerator driven system, which uses neutron spallation by a beam of high energy protons to fission actinides in a subcritical state. Although my son worked this summer at a neutron spallation facility, I'm not a fan of ADS reactors for various reasons, but what is interesting about this paper is the pyroprocessing of used nuclear fuels for separation of valuable elements therein.
From the paper's introduction:
Nitrogen-14, 14N, when bombarded with high energy neutrons, undergoes a nuclear reaction in which a proton is ejected from the nucleus, giving carbon-14, 14C. In general, most writers think that this is a bad thing, since carbon-14 is radioactive. However, I disagree. Carbon 14 has a very low neutron capture cross section compared to the two other carbon isotopes, the non-radioactive carbon-12 and the other non-radioactive (but rarer) isotope carbon-13. 14-C is a slightly less efficient moderator than natural carbon, meaning that regions of fast neutrons and epithermal neutrons are easier to maintain in breeder blankets where actinide 14-C carbides are used. I think we would be better off with more 14C, not less, but that's just my opinion.
As I've noted in this space, all of the transuranium isotopes have a critical mass in a fast neutron spectrum, and therefore it is not actually necessary to treat them with neutron spallation, as in an ADS system, but no matter. The processing is what is interesting, not the mechanism of fission.
Here's the flow chart for the process described:

There are several references to "waste" here. In my opinion, they are all unnecessary. Nothing that is useful is waste, and given the nature of our intractable chemical pollution problem, radiation can serve to remediate some very severe cases; in fact in some cases, it may be the only tool for remediation. I'd stay away from that zeolite thing, myself. Under these circumstances, this particular molten salt has a number of things that don't recommend it, one being the presence of chloride and the other being the problem of tritium being generated from lithium. (On the other hand, if people ever get fusion reactors to work, tritium will be a valuable fuel.)
To my way of thinking, this is not an ideal pyroprocessing approach, but it's nice one at which to look to stimulate thought.
A table of fuel composition on loading and on discharge:

Recovery of metals in the liquid cadmium cathode:

Decay heat:

The isotopic mix of the plutonium is actually quite wonderful. This is an example of proliferation-proof plutonium, because of it's heat load.
Nice stuff.
Esoteric, but this sort of thing represents the only path out of climate change in my opinion.
There are many, many, many other possible similar processing schemes. This is just one.
Enjoy your Friday.
John Quincy Adams. The first one I remember was Polk vs. Clay, and my first vote was for...
...Winfield Scott.
I was really pissed off when that asshole Franklin Pierce won the election, which lead to Buchanan...
Of course, Buchanan was, until Trump, considered the worst President ever, so it kind of gives one hope, since Lincoln came along and cleaned up the mess and actually made the country better than it was when those two asses before him held the office. We'll need another Lincoln, but we could do without Civil War II.
I note that the United States had a President before Trump who was also a traitor, albeit not to a foreign power as Trump is to Russia.
Ex-President John Tyler served in the Confederate government, and thus was a traitor to his country.
Premature mortality related to United States cross-state air pollution.
The paper I'll discuss in this post is this one: Premature mortality related to United States cross-state air pollution (Barrett et al., Nature 578, pages 261–265 (2020))
Ultimately the most dangerous fossil fuel wastes will be those associated with climate change, but dangerous fossil fuel waste has been killing people all over the world for a century. The de-industrialization of the United States, along with political laws associated with automotive and power plant emissions, the latter being part of the Clean Air Act now in the process of being gutted, and the substitution of dangerous natural gas for dangerous coal in the United States has lead overall to a decrease in the deaths here; we now export air pollution deaths to import trinkets for our bourgeois lifestyle, but people still die here from air pollution.
This paper is an attack on the ever popular theory advanced by the racists who dominated the Democratic Party in the 19th century, and in the big switch, the Republican party in the late 20th and early 21st century. It shows that it rains particles in the lungs in the just and unjust alike.
From the abstract, which is surely open sourced:
From the introductory text in the body of the paper:
Combustion emissions constitute the largest source of anthropogenic emissions in the USA, and therefore contribute to the formation of PM2.5 and ozone2. The health impacts attributable to these emissions have been estimated in various studies6,13,14, with estimates varying between 90,000 and 360,000 early deaths per year. In the context of the Environmental Protection Agency (EPA) Cross-State Air Pollution Rule (CSAPR) and individual state regulation, measures to further reduce the health impacts of pollution would benefit from a greater understanding of which sectors and which states are responsible for the health impacts in every other state.
The paper's goal:
Some graphics from the paper:
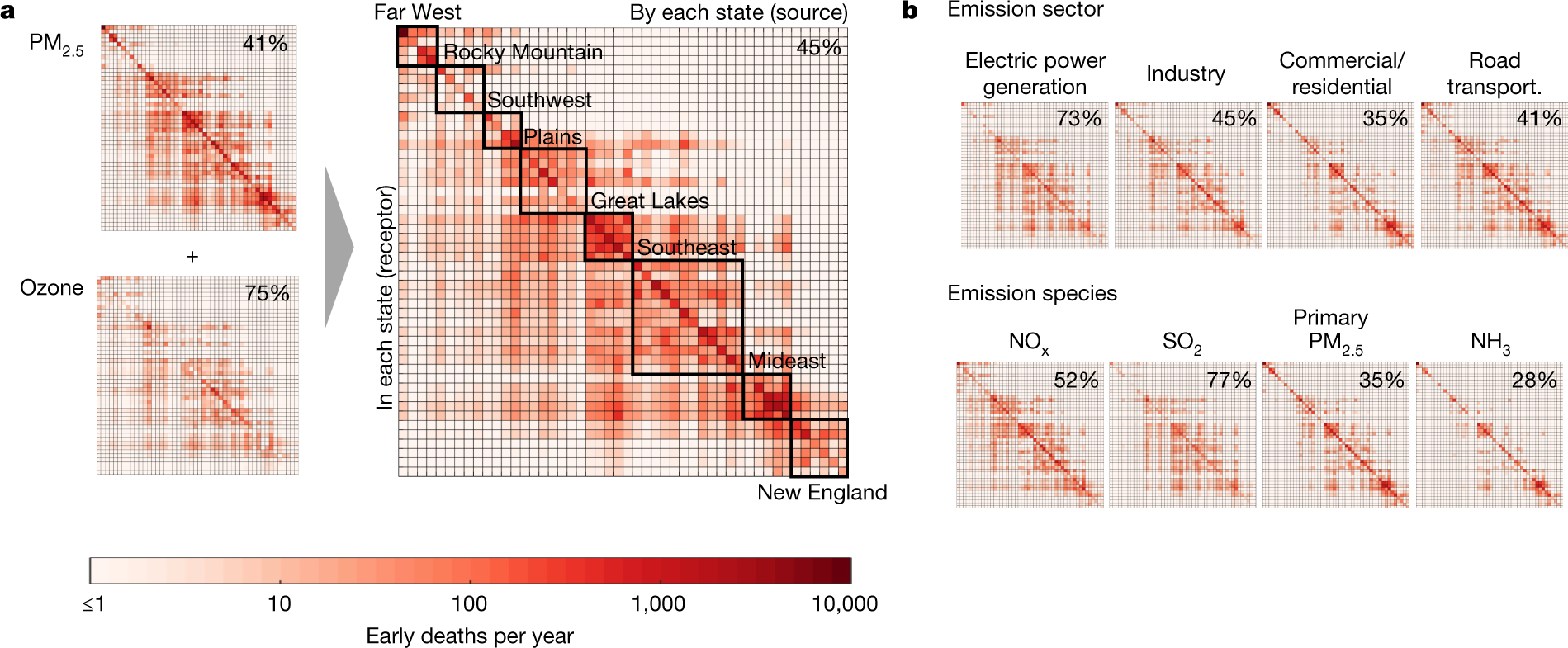
The caption:
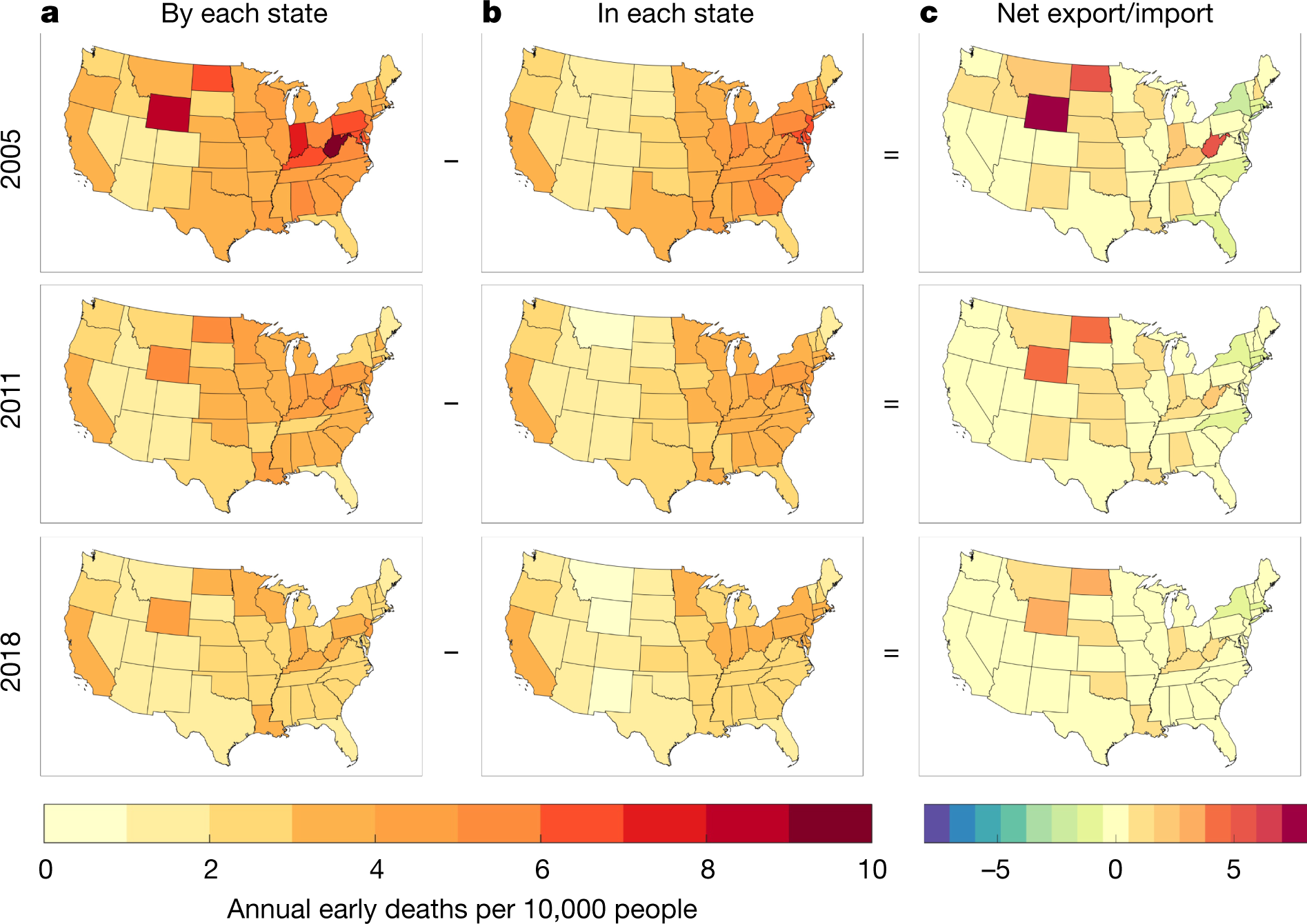
The caption:
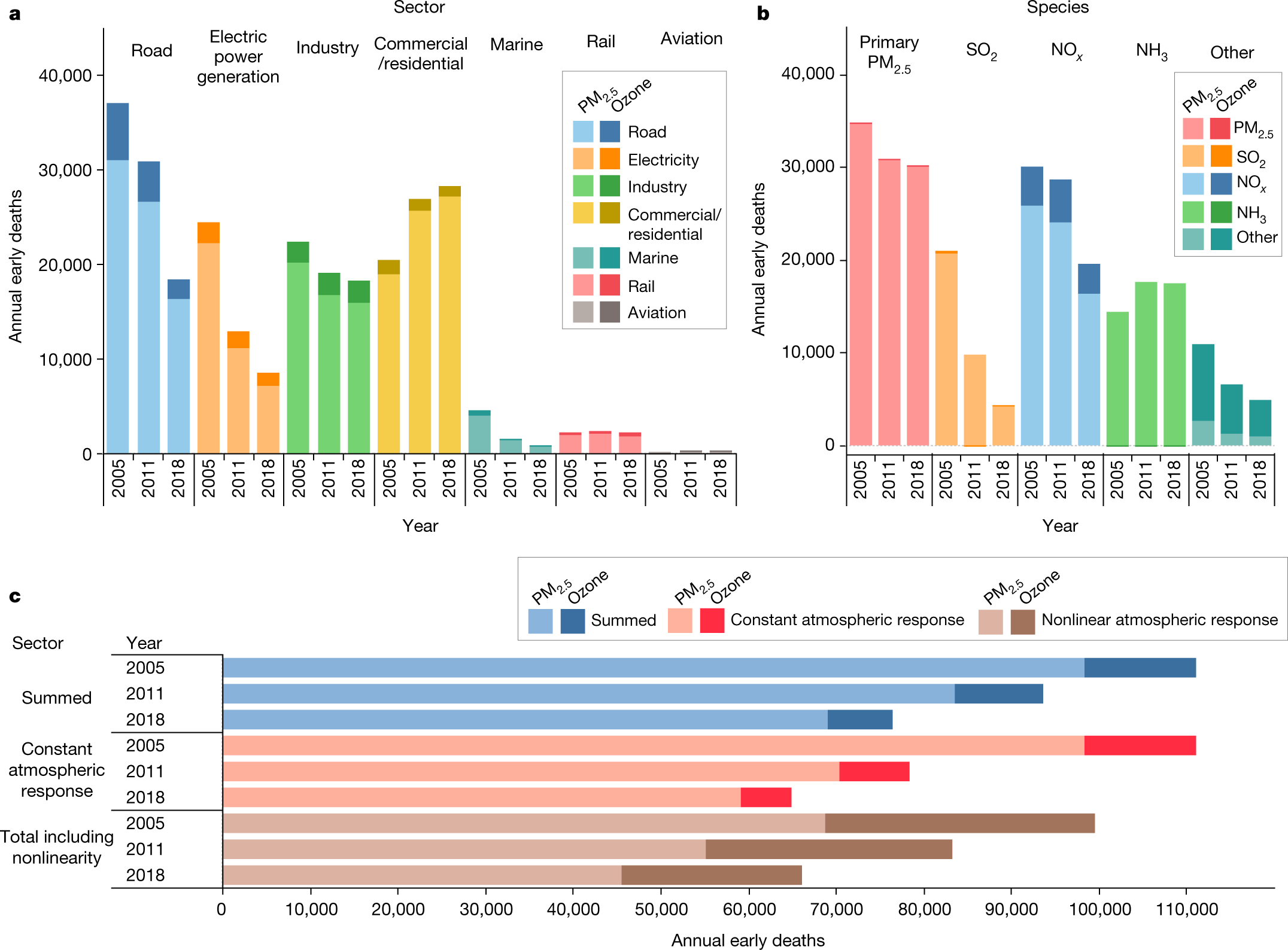
The caption:
Some more text:
Ammonia-attributable impacts increased by around 21% between 2005 and 2018. This difference was driven by an increase in the sensitivity of PM2.5 exposure with respect to a unit of ammonia emissions between 2005 and 2011. Owing to the decline in the importance of SO2, ammonia impacts went from being the fourth-greatest to the third-greatest contributor to total impacts over this period, increasingly close to the contribution of NOx species. NOx remained the second-greatest contributor to impacts from 2005 to 2018. Despite the roughly 50% reduction in total NOx emissions between 2005 and 2018, impacts attributable to NOx reduced by only around 35% between the two years. This is largely due to the increased sensitivity of PM2.5 formation to NOx emissions between 2005 and 2011, as noted previously23
Here in the United States we think that coal is dead because we've begun burning gas by utilizing a system by which we pulverize the bedrock of the continent irreversibly and in the process, probably destroy or severely damage the groundwater supply for all generations that come after us. Don't worry, be happy. We're green.
It is true that dangerous natural gas can cause particulates via Boudouard Chemistry, but overall the particulates are lower for dangerous natural gas than for dangerous coal. However the fastest growing source of energy on the planet as a whole - not that people in our racist country care about people in other countries where poor people make and/or recycle our precious stuff - has been coal.
We couldn't care less.
Don't worry; be happy. The steel for all those wind turbines we've bet the future of the planet on will be made in Chinese blast furnaces, and not in Pennsylvania. Here in New Jersey, we no longer have to breathe that shit from steel plants. They're closed and they're rotting. Pennsylvania just gives us our "clean" "transitional" natural gas now.
I trust you had a pleasant weekend. Mine was pretty good; not all the science I read was this depressing.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,515