NNadir
NNadir's JournalElectrochemical Production of Acetylene from CO2 Using Molten Chloride Salts.
The following paper is open to the public, and there is no need to excerpt it extensively: New Route of Acetylene Synthesis via Electrochemical Formation of Metal Carbides from CO2 in Chloride Melts Yuta Suzuki, Seiya Tanaka, Takashi Watanabe, Tomohiro Isogai, Akiyoshi Yamauchi, Yosuke Kishikawa, and Takuya Goto ACS Sustainable Chemistry & Engineering 2024 12 (5), 2110-2119.
It is well known that one route to acetylene is to react molten calcium metal with carbon and then hydrolyze it with water, generating calcium hydroxide and acetylene.
Acetylene is, of course, a fuel, generally used in welding torches and other high temperature applications, but it can also be a key intermediate in the production of ethylene (ethene) a very valuable chemical commodity.
What is cool about this paper is the production, electrochemically, of calcium (or lithium) carbide from CO2 in chloride melts.
By coincidence I just attended a lecture by the great chemist Jim Wishart of BNL on the subject of chloride melts, whereupon he commented on their structure, although I haven't necessarily been a chloride melt kind of guy, at least not for nuclear applications.
I commented on similar work designed to produce elemental carbon from CO2 elsewhere on DU:
Electrolysis of Lithium-Free Molten Carbonates
In a case where waste electricity is available as a result of process intensification using nuclear heat, these kinds of processes can be utilized, in theory, to reverse climate change. Note however that to reduce CO2, all of the energy generated that put it there, plus an investment of energy to overcome entropy, must be reproduced.
Nonetheless, this is a very cool paper, with nice references pointing to papers on the formation of valuable transition metal carbides.
Enjoy the work week.
Flax Processing for Those Who Like to Grow and Make Their Own Linen.
Just in case you lie awake at night thinking how to do this:
Grow your own linen.
I don't know how I get to these things, but I do: Flax processing.
A U.S. Lead Exposure Hotspots Analysis
The paper to which I'll refer in this post is this one: A U.S. Lead Exposure Hotspots Analysis Valerie G. Zartarian, Jianping Xue, Antonios G. Poulakos, Rogelio Tornero-Velez, Lindsay W. Stanek, Emily Snyder, Veronica Helms Garrison, Kathryn Egan, and Joseph G. Courtney Environmental Science & Technology 2024 58 (7), 3311-3321
The paper is open to the public, anyone can read it. I'll produce a few important excerpts on the "methods" which involves measurement of lead levels in children's blood but first produce here the telling graphic:

From the introduction:
Identifying and addressing remaining lead exposure risk hotspots are priorities in the United States. The Federal Lead Action Plan (10) and the U.S. Environmental Protection Agency (EPA) Lead Strategy (e.g., Goal 2, “Identify Communities with High Lead Exposures and Improve Their Health Outcomes”) (9) highlight the need for lead mapping as part of whole-of-government efforts to address high exposure risk locations and disparities. Data mapping can inform screening and prioritization efforts to guide interventions and “deeper dive” analyses (such as enhancing children’s blood lead level (BLL) surveillance data analyses and lead source apportionment analyses). These analyses can assist in efforts around primary prevention; lead-based paint mitigation; lead remediation, enforcement, education, and outreach. (11) Federal agencies are collaborating to identify geographic locations and populations at risk for lead exposure so that they can be addressed proactively. Examples include targeting HUD remediation grants, EPA environmental cleanup actions, and CDC primary prevention and enhanced blood lead testing programs for children. (5)
The methods:
1. Statistically evaluated hotspots identified with lead indices against children’s BLL surveillance data:
(a) from Michigan (MI) and Ohio (OH), using the BLL data and statistical methods described in Xue et al. (12) and Stanek et al., (13) respectively, and an expanded set of national lead indices;
(b) from matching hotspots identified using lead indices with community hotspots identified in 9 state health department public reports (listed in Zartarian et al. (5)) and quantifying the percent; a match is defined here as a community with at least one census tract identified by the lead indices in our analyses;
2. Compared existing national indices against each other and against available BLL surveillance data using sensitivity, specificity, and Cohen’s kappa score to determine which indices are the statistically strongest predictors of hotspots for the national-scale analysis;
3. Produced census tract-level maps for the United States that visualize the intersection and collective combination of hotspots based on the two methods discussed in Xue et al., (12) top 20 (i.e., 80th–100th) percentiles and Getis-Ord Gi* (14) geospatial cluster hotspots analysis methods;
4. Conducted national-scale analyses to identify states and counties with the highest potential lead exposure risk, based on the considered indices and the number of children younger than six years old in the identified 2010 census tracts (n = 73,086 census tracts containing at least one child less than 6 years old in the 50 states)...
I added the bold. "BLL" refers to "blood lead levels"
I had a rather long riff on lead exposure here:
For my 30,000th post, I'd like to thank DU for inspiring me to expand my knowledge, and of course...
Have a pleasant Sunday.
At the Mauna Loa CO2 Observatory, Yet Another Terrifying, Startling Week in 2024.
Two weeks ago I interrupted my standard language for my "monitoring the collapse of the atmosphere through the Mauna Loa CO2 observatory" posts, to remark on being incredibly shocked by the numbers in 2024.
That post is here: At the Mauna Loa CO2 Observatory, a Terrifying, Startling Week and Month, New Records Everywhere.
I also had a thread with a correction to statements I made in that post: An Illuminating Error in My Recent Terrifying Mauna Loa Post.
The point of the second post, the "Illumination" is that 2024 is shaping up to be worse than the worst year ever at the Observatory ever, 2016. Regrettably that trend continues. The 2nd worst week ever in year to year comparators of weeks of the year with that of its previous year has just been observed, just two weeks after the worst was observed.
The week beginning February 18, 2024 is 5.43 ppm higher than the week beginning February 19, 2023.
Before I discuss the terrifying details of yet another astounding number in 2024, here's the standard language I use in these posts:
Facts matter.
When writing these depressing repeating posts about new records being set, reminiscent, over the years, to the ticking of a clock at a deathwatch, I often repeat some of the language from a previous post on this awful series, as I am doing here with some modifications. It saves time.
A recent post of this nature is here: At the Mauna Loa CO2 Observatory, 2024 Starts With a Fairly Disgusting Bang.
As I've been reporting over the years in various contexts, the concentrations of the dangerous fossil fuel waste carbon dioxide which is killing the planet fluctuate sinusoidally over the year, with the rough sine wave superimposed on a roughly quadratic axis:
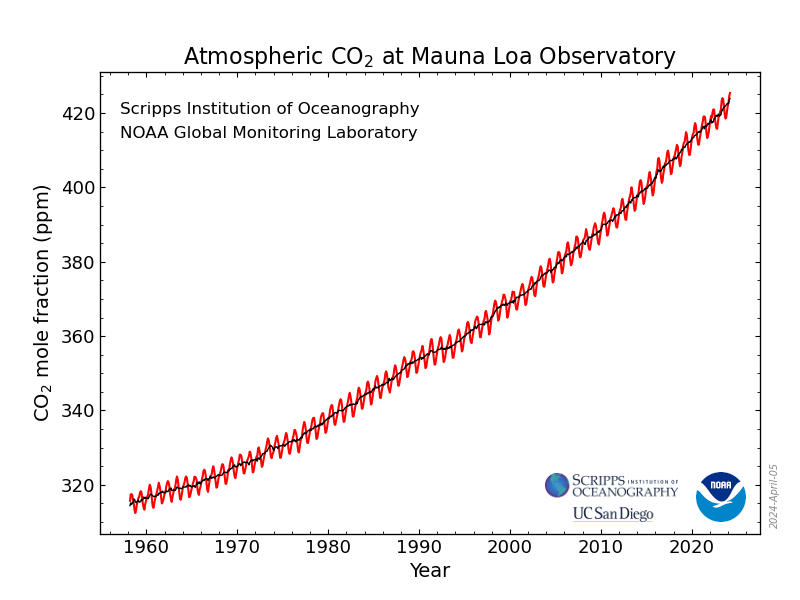
Monthly Average Mauna Loa CO2
The Observatory posts on its data pages curated and reviewed averages for daily, weekly, monthly, and annual data. I maintain spreadsheets for the latter three to use in calculations.
The data for the week just ended, the week beginning February 18, 2024:
Week beginning on February 18, 2024: 425.11 ppm
Weekly value from 1 year ago: 419.68 ppm
Weekly value from 10 years ago: 398.53 ppm
Last updated: February 24, 2024
Weekly average CO2 at Mauna Loa
First and foremost. 425.11 ppm is the 2nd highest value ever reported at Mauna Loa for the concentration of the dangerous fossil fuel waste carbon dioxide in the planetary atmosphere, slightly less than the value recorded two weeks ago 525.83 ppm. (There is some local "noise" in these readings.)
There have been 2507 points of this type recorded going back to 1975.
This week's increase, from week 5 of 2023 to week 7 is an astounding 5.43 ppm, the 2nd greatest such week to week comparison between consecutive years ever record, by far, the (now) 3rd worst ever having occurred in week beginning July 31, 2016, when the increase was 5.01 ppm, making it one of only three to exceed a 5.00 ppm increase, two from this year, 2024, and one from 2016. 2024 is shaping up to blow 2016 out of the water as "worst ever."
The global average going back to 1975 for all 2507 weeks reported is 1.87 ppm increases in year to year weekly comparators. In the 21st century, that average is 2.20 ppm increases for all weeks in the 21st century.
There have been only seven readings for any of the 2507 weeks in the database that exceeded 424 ppm, all since January 1st, 2023, five in 2023, and two in 2024, a year which is still young. The only readings to exceed 425 ppm have come in 2024. The annual peak will take place in April or May, at the latest in early June. It is sure to be an astounding and terrifying number.
Of the top 50 highest readings out of the 2507, 12 have taken place in the last 5 years, 33 in the last 10 years, and 40 this century.
Of the ten highest comparative year to year readings that took place in the 20th century; six are found in positions 40 to 50.
Three of the first seven weeks of 2024 compared to 2023 appear in the top 50 ever observed.
The week to week comparators from ten years ago (comparators to the same week, week 7, of 2024 to that of 2014) shows an increase of 26.58 ppm, the second highest such increase ever observed after that of two weeks ago, 27.65 ppm higher than week 5 of 2014. A 52 week running average of 10 year comparators, which sort of smooths out the "noise" has reached 24.62 ppm/10 years, the highest value ever. In the seventh week of 2001, that running average was 15.25 ppm/10 years.
I am now maintaining a spreadsheet of the daily readings at the Mauna Loa CO2. Of the top 10 readings ever going back to 1975, seven have taken place in the month of February 2024, one in January of 2024, and two in 2023. The two highest were over 426 ppm.
In spite of these ever worsening and ever more astounding numbers - people lie to each other and to themselves but numbers don't lie - you will still find people mindlessly cheering for bourgeois toys that do nothing, be they electric cars, solar cells and/or wind turbines, to address climate change, all of which are exercises in promoting the use of fossil fuels, the destruction of wilderness, and the demand for mining.
The big lie people tell themselves and each other that this pixilated reactionary scheme, electric cars, solar cells, wind turbines, blah, blah, blah is "doing something" about climate change. This is nonsense. That it is nonsense is clearly shown, again, by the numbers. The reactionary scheme of carrying on about so called "renewable energy" that led us here was never about climate change or any other environmental issue and the claim that it is is an afterthought. It was always about attacking the only realistic alternative to fossil fuels, nuclear energy.
The antinukes won and humanity, and in general the rest of the biosphere lost.
We're clueless.
Enjoy the weekend.
Quantifying the Photolytic Fate of Fluorinated Pharmaceuticals and Pesticides.
The paper I'll discuss in this post is this one: Fluorinated Pharmaceutical and Pesticide Photolysis: Investigating Reactivity and Identifying Fluorinated Products by Combining Computational Chemistry, 19F NMR, and Mass Spectrometry Akash P. Bhat, William C. K. Pomerantz, and William A. Arnold Environmental Science & Technology 2024 58 (7), 3437-3448
The extreme stability of the carbon fluorine bond has made it persistent in the environment, and has resulted in the serious pollution of all terrestrial matrices on this planet, including but not limited to biological matrices. Garnering most attention are the compounds related to consumer and industrial products such as Teflon, certain upholstery protection products, lubricants, coatings, and fire fighting foaming agents, collectively know as "PFAS." One of the concerns connected with the toxicology of the compounds concerns the fact that fluorine in organic molecules has properties that mimic hydroxyl functions, -OH groups, that are very common in all of the major biologically active classes of molecules, notably sugars, including those involved with DNA and RNA, proteins, in particular those rich in the residues serine, threonine and tyrosine, and even certain important lipids signaling, sphingosines, for one example. Fluorine, like oxygen, can form hydrogen bonds, and thus can interfere with biological processes. In addition, trifluoromethyl and difluoromethyl groups are electron withdrawing, and thus can play a role in mimicking the ubitiquous carboxylic acids in living systems. Combined with their stability, which can vastly increase their resistance to metabolism, fluorocarbon moieties are often used by agrochemists and medicinal chemists to lengthen the lifetime and activity of pesticides and medications. Because these compounds are either deliberately sprayed into the environment in the case of pesticides, or excreted in urine in the case of medications, either intact or partially metabolized, and thus find their way into rivers, streams and other bodies of water in the outfall pipes of sewage treatment plants, or groundwater from septic systems, they can persist for a very long time, adding to the PFAS problem, in particular because these molecules are designed for biological activity.
The authors here focus on the mechanism of degradation of fluorinated compounds using radiation, and explore analytical chemistry techniques in following degradation products.
From the introduction to the paper:
The most common method used for identification of fluorinated degradation products of pesticides and pharmaceuticals is liquid chromatography with nontargeted high resolution mass spectrometry (LC-HRMS). (17) It was reported that using only LC-HRMS to quantify fluorinated compounds can result in fluorine mass balances falling short, with up to 90% of fluorine being unaccounted for. (17?20) Recent studies on fluorinated product formation and identification during photolysis and advanced treatment processes have reported more complete product identification by complementing LC-HRMS with quantitative 19,F-nuclear magnetic resonance spectroscopy (NMR) analysis. (9?11,21,22) An NMR spectrum contains all fluorinated product peaks, and the chemical shifts provide insight into the identity of the functional group and its chemical environment. With the broad range of fluorine NMR shifts (?400 ppm) and the 100% abundance of 199F isotope of fluorine in the environment, 19F NMR has been reported to be a highly suitable method to detect fluorinated compounds and products. (23) Also, by increasing the number of scans four times, the signal-to-noise ratio is doubled, allowing minor products to be detected. Using 19F NMR to quantify the products formed assists in evaluation of chemical structures identified using the semiquantitative (at best) LC-HRMS data. (10) For example, the photolysis of fluoxetine led to formation of norfluoxetine, TFA, and fluoride. (10) On the other hand, the 19F NMR spectra after photolysis of sulfoxaflor showed that all product peaks formed were similar in shift to the parent peak, indicating that all products were closely related to each other and the parent structurally, making evaluation of product data obtained via LC-HRMS simpler, faster, and more precise. (9) In cases such as this, however, where multiple 19F NMR peaks are within several ppm the parent peak, it is difficult to assign these NMR peaks to the specific structures identified by LC-HRMS to have modifications remote to the fluorinated functional group of interest, which hinders quantifying the relative amounts of different products. (24)
The 19F NMR shifts for common products like fluoride (F–), trifluoroacetate (TFA), and difluoroacetate (DFA) are known and are easily identified and quantified. For new fluorinated product structures formed via degradation of pesticides and pharmaceuticals, it is difficult to predict the upfield or downfield movement of 19F NMR shifts with respect to the parent compounds, thereby potentially making accurate matches of products identified using LC-HRMS with the NMR data challenging and complicated. Major products may go unidentified, unless these products are matched to their 19F NMR shifts. With the unavailability of mass-labeled standards for quantitative mass spectrometry for these newly identified fluorinated products and the difficulty in separating products using preparative scale chromatography, computational calculations for 19F NMR shifts could pave the way for precise and accurate identification of products leading to matches between experimental 19F NMR spectra and LC-HRMS product structures.
Computational methods can be used to obtain 19F NMR shifts for fluorinated organic compounds, even in the absence of standards...
The synthetic difficulty of making PFAS standards is, by the way, profound, and 19F NMR as the authors note can overcome these problems, albeit at the expense of sensitivity.
A graphic in the article gives examples of commercial compounds which feature fluorinated carbons:
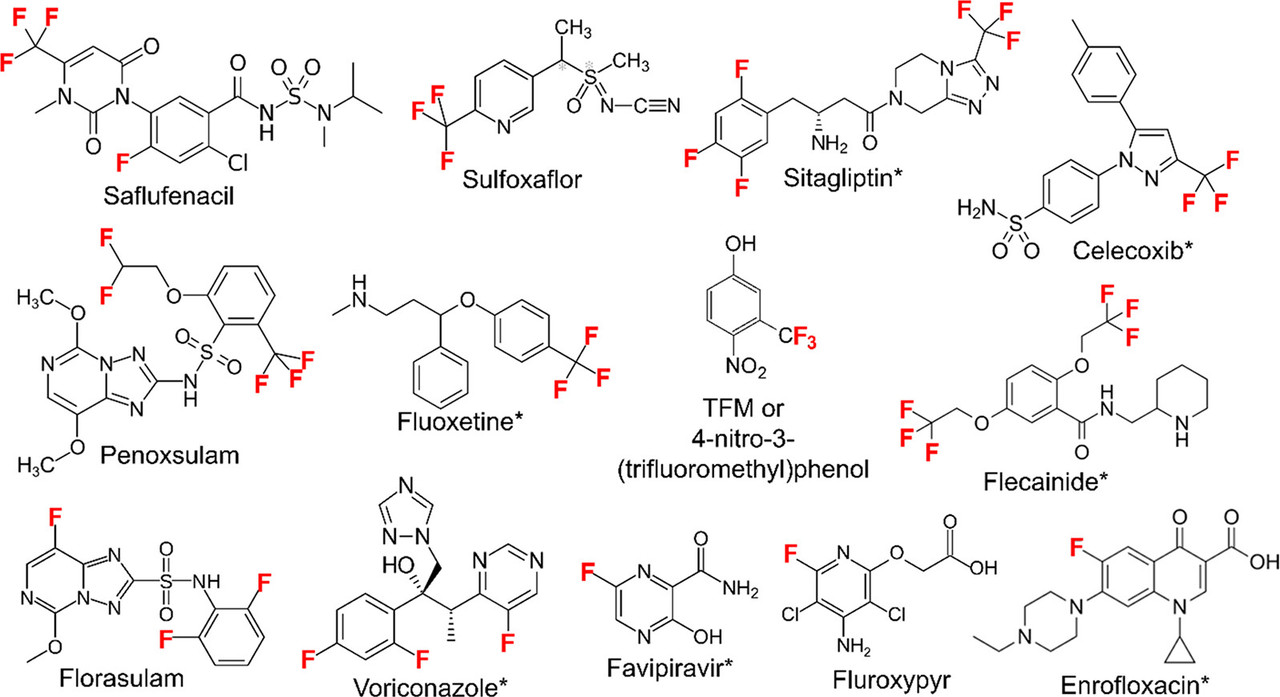
The caption:
The radiation used by the authors to study the degradation are as follows: 255, 275, 308, 365, and 405 nm, provided by LEDs and mercury lamps. All of these wavelengths, except the visible violet 405 nm are in the UV range as opposed to the visual range. Our wonderful industrial and agricultural efforts to destroy the ozone layer means that we can be assured of the degradation of these molecules over the long run, albeit at the expense of limiting or completely destroying life on Earth. (Problem solved?)
The results of the experiments and the products are shown graphically in the rather lengthy supplemental information, and happily in some cases, one of the products is the fluoride ion but other fluorinated species, notably the problematic TFA, trifluoroacetic acid, is well represented. Shorter wavelengths, unsurprisingly, are more efficient in general in producing the fluoride ion it seems.
An interesting paper.
Have a nice weekend.
British company pioneers new nuclear welding technique
If we have any hope of addressing climate change, increasingly a long shot, we will need to scale up nuclear energy much faster than even the remarkable pace at which China is now putting out new nuclear reactors, a pace matching that of the United States in the 1960's and 1970's, when the US built more than 100 nuclear power reactors in less than 25 years. The result left the United States the world's largest producer of nuclear energy up to this day. Starting almost from zero in 2000, China has surpassed France as the world's second largest producer of nuclear energy after the United States.
As my son is involved in nuclear metallurgy, and works with ion beams in labs working on 3D printing of reactor cores, and in fact because my son received his Masters degree in work involving welding, this high speed technology for ion beam welding to reduce the time required for welds, this article immediately caught my eye
British company pioneers new nuclear welding technique
Subtitle:
Some excerpts of the short article:
Sheffield Forgemasters deployed specially developed parameters, meticulously fine-tuned during the welding development stage, including innovative sloping-in and sloping-out techniques to start and finish the weld, ensuring a clean and complete weld-join.
"We are delighted to have reached a significant milestone in assembling a nuclear vessel demonstrator, using electron beam welding for the first time at this scale, with 100% success and no defects," said Jesus Talamantes-Silva, research, design and technology director at Sheffield Forgemasters.
Michael Blackmore, senior development engineer and project lead, added: "The implication of this technology within the nuclear industry is monumental, potentially taking high-cost welding processes out of the equation.
"Not only does this reduce the need for weld-inspections, because the weld-join replicates the parent material, but it could also dramatically speed up the roll-out of SMR reactors across the UK and beyond, that's how disruptive the LEBW breakthrough is."
Sheffield Forgemasters - the only company in the UK with the capability to manufacture the large forgings required for SMRs - said the demonstration of LEBW technology's potential opens new horizons for "more efficient, low-cost and less time-heavy nuclear assemblies" and also has far-reaching implications for other projects which require thick-walled welded assemblies... "With a diameter of three metres and a wall thickness of 200mm, construction of the vessel showcases the reliability and capabilities of LEBW, setting a dramatic new standard for weld-joining thick-walled components, previously untrialled in a demonstrator model," the company said.
Sheffield Forgemasters deployed specially developed parameters, meticulously fine-tuned during the welding development stage, including innovative sloping-in and sloping-out techniques to start and finish the weld, ensuring a clean and complete weld-join.
"We are delighted to have reached a significant milestone in assembling a nuclear vessel demonstrator, using electron beam welding for the first time at this scale, with 100% success and no defects," said Jesus Talamantes-Silva, research, design and technology director at Sheffield Forgemasters....
If verified, reportedly this technology will reduce the time required for weld inspections.
When the French nuclear industry was being drained of funding and used as a cash cow for a misguided attempt to replace the magnificent French nuclear industry with rickety so called "renewable energy," problems with welds in the reactors were observed and the infrastructure fell into decay and disuse while the problems were corrected. After two years, to reengage the fight against climate change, the French nuclear industry is well on its way to recovery, with production approaching earlier levels and profitability. This said, we have to do far more to address climate change than simply keeping our current reactors going; we have to exceed the remarkable Chinese pace world wide in building new reactors.
This British technology is a step in the right direction.
There is a photograph of the core subject to these novel welds at the link to the full article, which is free to read.
Recovering Lanthanides (Rare Earths) From Acid Mine Drainage.
Recovery of Rare Earth Elements from Acid Mine Drainage with Supported Liquid Membranes: Impacts of Feedstock Composition for Extraction Performance Andrew Middleton, Benjamin C. Hedin, and Heileen Hsu-Kim Environmental Science & Technology 2024 58 (6), 2998-3006.Over the last several months, as papers on the subject pile up, happily at an increasing pace, on the subject of removing uranium from aqueous matrices, seawater and groundwater and to a lesser extent, riverine water, where it often occurs, in all three places, naturally as an example of "NORM" (naturally occurring radioactive materials), as well as from anthropogenic sources, uranium mine associated runoff, reprocessing sites generally devoted to weapons manufacture, and coal ash, in which uranium is often a prominent constituent, I have been working on a long post to discuss these many interesting approaches to the idea. Most of these are based on resins, often functionalized with amidoximes, but many other approaches are also discussed in the literature, extending even to proteins found in corals that are known to complex - for what evolutionary purpose I do not know - uranium. I may never get around to publishing it, but I often write posts at DU to force myself to look at the details, as opposed to superficial notation, of particular technologies devoted to a sustainable world, the world which, unfortunately, we do not live, but is, I believe, feasible to create, if unlikely given our mythology driven world culture.
I will not live to see a sustainable world, but hopefully I can hand off some of these ideas, if they have merit, to those who will follow and live with the environmental disaster now well underway.
I often write, in terms of energy, of "process intensification" wherein matrices at high temperatures provided by nuclear fuels are cooled in multiple processes that recover useful products and goals, clean desalinated water, clean portable fluid fuels (DME generally), landfill elimination, and downstream, as a side product, electricity. The idea is that if one can achieve multiple goals with one process or device, one should do so; this is the essence, to my mind, of sustainability.
One of the things we are leaving to the unfortunate generations who will follow us, is mine waste. We of course, consider this waste, but our future generations may have to pick through our garbage merely to survive, as we will have impoverished them. We generally think of mine waste in terms of tailings - some of which contain elements for which we have only recently found important industrial uses, indium for example in zinc mine tailings - but contaminated water is another product of mines.
As far as mines go, things will get much worse before they get better. It is a popular, albeit dangerous, reactionary and rather stupid idea, that so called "renewable energy" is sustainable. It isn't. The term "renewable energy" is essentially an oxymoron: The land intensity and the mass intensity driven by short lifetimes, low energy to mass ratios, and the need for redundancy to address the inherent lack of reliability of the weather - particularly in a period where we have dangerously destabilized the weather - make "renewable energy" not only lack sustainability, but is actively driving the acceleration of environmental degradation. So called "renewable energy" is nothing more than a highly questionable promotion on dependence on mining on land, and now we see, with Norway's recent disastrous decision, at sea.
I am thoroughly convinced of this, despite the unpopularity of my immutable opinion.
It is a huge mistake to confuse popularity with wisdom.
Among the many mined materials on which the so called "renewable energy" industry depends - it is hardly limited to so called "renewable energy" but extends to many other industries - are the lanthanides, in popular parlance, "the rare earth" elements. As is widely discussed in the critical materials issue in academic, industrial and government circles, the world overwhelmingly relies on Chinese mines for these elements, and there is a huge effort, worldwide, to source them elsewhere. The paper at the outset considers recovering them from a well known (and growing) source of water pollution, "acid mine drainage."
From the introductory text of the article:
AMD is generated from the dissolution and oxidation of sulfide minerals in waste rock piles and exposed rock formations produced during mining activities. Water that continuously leaches from these mined areas is enriched in soluble minerals and metals such as REEs, creating a potential source of critical metals. (8,9,14) In the northern Appalachia region of the United States alone, hundreds of abandoned coal mines collectively release 500–3400 t of REE annually. (9,15,16) While the total loading of REE into watersheds is large, the aqueous concentrations of REEs can be relatively low. AMD fluids with the highest concentrations of REE tend to have low pH (i.e., less than pH 4). (9) Even for these low-pH and REE-enriched AMD fluids, the aqueous composition is complex: total REE concentrations are exceeded by up to 5 orders of magnitude by major metals such as Fe, Al, Ca, and Mn. (8) As such, the development of separation processes for AMD and other low-grade feedstocks requires an understanding of the impact of these impurities and other water quality variables on REE recovery processes.
Separations by SLMs are a modification of solvent extraction, where the extraction and stripping processes have been combined into one unit operation. In SLM separations, the feedstock and product solutions are separated by a hydrophobic membrane that has been impregnated with an organic extraction solvent, such as di(2-ethylhexyl)phosphoric acid (DEHPA), dissolved in an oil phase...
Let's stop here. These "SLM" (Supported liquid membranes) are made by soaking a polyvinylidene fluoride, a polymer that is related to teflon, in kerosene containing a lanthanide complexing agent used in the chemical extraction of lanthanides, di(2-ethylhexyl)phosphoric acid (DEHPA). These are placed directly into samples of the contaminated water for the experiments, but not in the flowing mine drainage streams. This information may impact the environmental profile of this particular scheme for using a liquid membrane, but the reader should be aware that there are many other approaches besides "SLM" or even this particular variety of an "SLM;" the important issue is the quantity of elements that can be theoretically (or even practically) recovered from acid mine drainage, not the details.
The authors select, as their test sites shown here:

The caption:
The metals and other species in the water at the seven abandoned mines are described in a table in the paper which follows:
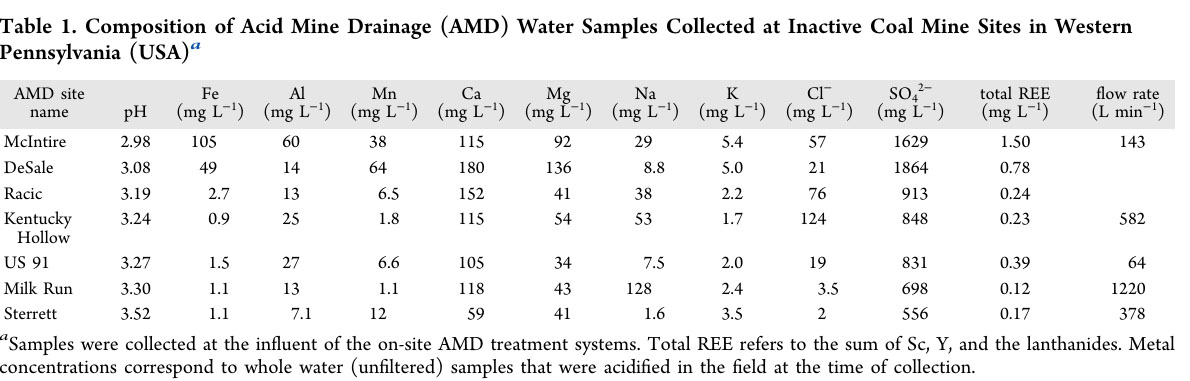
One should note that the amount of lanthanides and the two congeners that are found with them, scandium and yttrium, which are not "f elements" but rather "d elements" but are always found with the lanthanides, is dwarfed by the amount of iron, manganese, and in particular calcium and sodium.
I have loaded the data in this table into a spread sheet, and from the flow rates available for five of the seven mines - flow rates at the Desale and Racic mines are not reported - I have produced an estimation of how much of each of the cationic (metallic) elements flow in a year.
Approximately 3300 tons of iron flow out of the five mines in a year.
Approximately 8300 tons of aluminum flow out of the five mines in a year.
Approximately 2500 tons of manganese flow out of the five mines in a year.
Approximately 49,000 tons of calcium flow out of the five mines in a year.
Approximately 22,000 tons of magnesium flow out of the five mines in a year.
Approximately 39,000 tons of sodium flow out of the five mines in a year.
Approximately 1200 tons of potassium flow out of the five mines in a year.
The fourteen lanthanide elements plus yttrium and scandium total 221 tons out of the five mines in a year.
The supplementary information of the paper gives the breakdown of the 16 elements. Between 45% and 51% are represented by just two elements in the separate mine drainage, yttrium and cerium. Between 63% and 66% are represented by adding neodymium to these two, between 71% and 77% are covered by adding lanthanum to these three, and between 77% and 81% are covered by adding gadolinium.
All of these elements are useful and have markets, but they are not quite as critical as some of the others, in particular, dysprosium.
I have discussed dysprosium here:
Material Flow Analysis of Dysprosium in the United States
...and here:
Uncovering the Key Features of Dysprosium Flows and Stocks in China
If China is concerned about dysprosium availability, everyone should be concerned about it.
Overwhelmingly modern generators and electric motors utilize neodymium-iron-boride magnets, which heat up in use, accounting for some of the thermodynamic inefficiencies of generators and electric motors. Heat can degrade the quality of the magnetic fields of permanent magnets, and so, to stabilize the magnetic strength of iron, small amounts of dysprosium is added to them to stabilize their magnetism under high temperature conditions.
One hears of an "energy transition" frequently as if one exists. It doesn't exist; we are more dependent on dangerous fossil fuels than we have ever been. One hears that dysprosium is a critical element for the mythical "energy transition" and certainly the quixotic quest for so called "renewable energy" does raise demand for dysprosium in wind turbines, where it is stranded when the wind isn't blowing, but generators in dangerous coal powered plants, dangerous natural gas powered plants, and dangerous petroleum powered plants also use dysprosium laced magnets, as do the magnets in hair blowers, fans, electric cars, drink mixers, audio speakers, etc., etc., etc., on and on and on. One can, of course, make magnets from say, just iron, but the cost of doing so will be environmental, the energy efficiency of all of these devices will be lower and thus the environmental impact higher.
The five mines studies leach out about 600 kg, less than a ton, of dysprosium every year. The price of dysprosium in recent times has varied between roughly $400/kg and $750/kg in recent years, but one should note that this represents isolated dysprosium, separated from all of the other 15 elements in the lanthanide fraction including yttrium and scandium. In fact most of the economic value of the lanthanides lies with dysprosium, neodymium, and praseodymium.
Dysprosium demand is roughly about 8000 tons per year.
It is thus doubtful that the cost of recovery systems for these elements can be covered by their sale, but this may not be the main reason for accomplishing the recovery. The reason for removing the metals might be something that is often neglected in discussions of the embrace of technology which are generally only addressed in purely materialistic terms, cost. For instance, advocates of the failed and useless solar and wind industries often like to crow that they're "cheap" even though they require redundant systems which default to the use of dangerous fossil fuels, albeit coupled with soothsaying about vast mining enterprises in the future. (Mines depend on fossil fuels.) Thus the real cost, known as the external cost, the cost to the environment and human health is treated as if it doesn't exist, hence 7 million air pollution deaths a year and a planet in flames. Wind and solar are not "cheap" even if the electricity produced by them for short periods cannot be sold at a decent price, and in fact, no electricity can be sold at a decent and sustainable price, insuring that everybody loses.
Thus I would like to suggest that the recovery of elements from dilute sources like acid mine waste be undertaken for a different reason than satisfying bourgeois affectations: Decency. Future generations will be required to recover metals from dilute sources, waste heaps, and things like this mines. When we mutter obscenely about putative nirvanas (that never arrive) "by 2000" and then when 2000 comes, "by 2020" and then when 2020 comes "by 2040" and so on ad infinitum, what we are saying is that future generations should do what we have not bothered to do ourselves. Hey folks, rather than foist responsibility on our grandchildren, how about we take action ourselves. The side product of removing metals from streams of water is clean water.
This is the same as is the case with uranium. It is cheaper to mine uranium than to recover it from seawater, or from water supplies either naturally or as the result of anthropogenic isolation and processing. However if we remove uranium from natural resources, then we need to mine less or perhaps not mine any at all.
I do not expect an ethical world; at the end of my life, experience teaches me that expecting ethics to dominate decision making is a fool's errand, particularly in the age of the triumph of blank indifferent materialism. This said, an ethical world is feasible if not likely, and thus I like the focus of this paper.
Have a nice evening.
Video graphic, growth and leading nations in nuclear energy 1966-2022
China is now #2. One should note the length of time it took China to go from number zero to number 2, or for that matter, the amount of time it took for the US to go to #1.
OK. I finally did it. I watched 15 minutes of "Jersey Shore."
My son (genetically approximately 1/4 of his genes are of Italian origin) has a new girlfriend, a fellow nuclear engineering graduate student. On learning that he's from New Jersey, where she's never been, she asked if it's really like "Jersey Shore."
We never saw the show, so I rented it, and watched 15 minutes of the first episode with my wife (who grew up on Staten Island).
We laughed a bit, then turned it off.
It was apparently about being proud of being a "Guido," or a "Guidette," body building, considering one's self as incredibly sexy, and drinking. The show is about being young, clueless Americans of Italian extraction who happen to congregate in New Jersey.
Of course, when I was growing up on Long Island, I thought of New Jersey as some kind of hellhole, an opinion I continued to hold when I lived in California, until I moved here.
We were embarrassed because we actually brought our kids to Seaside Heights for family vacations. Trust me, it wasn't like that.
I suppose somewhere in this state there is this kind of reality, but no, that's not us.
New Jersey is a kind of heaven. It takes a while to realize this, but it's true.
The Case for Risk Based Environmental Analysis of Floating Solar Cells.
The paper to which I'll refer in this post is this one: Making a Case for Environmental Risk-Based Monitoring of Floating Solar Systems
Mainak Bhattacharya, Arun Kumar, Arvind Kumar Nema, Sovik Das, and Arya Vijayanandan Environmental Science & Technology 2024 58 (6), 2595-2597
I make no secret of my abhorrence of so called "renewable energy" because of its unacceptable energy to mass ratio, its unreliability, its dependence on fossil fuels, and the bizarre definition by many people calling for the industrialization of wilderness for short lived consumer junk as "green."
I often note that while people have recently begun, despite the data showing its uselessness, to claim that affection or affectation for so called "renewable energy" is about climate change. It is no such thing. The mindless embrace of "renewable energy" has done nothing to address climate change. As I often note, the more money and resources that are squandered on renewable energy - in this waste is growing, not falling - the faster the degradation of the atmosphere is proceeding.
Numbers don't lie: At the Mauna Loa CO2 Observatory, a Terrifying, Startling Week and Month, New Records Everywhere.
One still sees dubious affection for so called "renewable energy" in the scientific literature - which is after all a human enterprise, in no way oracular although guided toward truth by process, but still subject to human flaws - but I have noted increasing questioning of the enterprise in recent years, based on the realities that this reactionary program are now presenting.
Having destroyed and decimated much land mass, now there is active discussion of destroying bodies of water for this affectation, so I welcome this paper's immediate questioning of the enterprise, looking before we leap. We already have wind turbines at sea, the plastic coating on the vanes being known to peel into microplastics, and now we want to add plastic floats for wind turbines.
The authors of this paper note the risks of this ongoing nonsense:
The FPVC typically consist of (1) floating pontoons made of materials like polyethylene (PE), low-density polyethylene (LDPE), high-density polyethylene (HDPE), and pyrocarbon; (2) floating electrical sensors and cables and other electronic equipment covered with PE-type materials that contain trace quantities of heavy metals (Al, As, Cu, Mn, Ni, and Zn) and boron; (3) partially or fully submerged anchors and moors made of HDPE and concrete; and (4) tilted solar panels that are not in direct contact with water but occasionally touch the host water body during high tides, heavy winds, rainfall, washing, accidental breakage of solar panels etc. The composition of the solar panels varies depending on their types. The first- and second-generation solar panels mostly used in the energy sector contain Si, Al, Ag, Pb, Cu, Zn, Cd, In, Ga, Se, Mo, and Te. With respect to all of the aforementioned information, three major environmental concerns might arise. (1) Penetration of sunlight into the host water bodies will be hampered, which will subsequently decrease the chlorophyll content and algal population that might further affect the overall ecosystem of the water body. (2) The dissolved oxygen (DO) concentration below the floating solar panel might decrease, which can affect the aquatic biota. (3) Leaching of microplastics, organic carbon, heavy metals, and metalloids from different components of FPVC might cause contamination in the host surface water body (Figure 1). All of these aspects, along with the steps required for systematic management of FPVC projects, are discussed further here...
The key to getting a question answered is to ask it. I applaud these authors for doing so.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,568