Science
Related: About this forumThe Rise In Nitrite Oxidizing Enzymes in Oxygen Depleted Open Ocean, Iron Demand, and the Atmosphere
The paper I'll discuss in this post is this one: Abundant nitrite-oxidizing metalloenzymes in the mesopelagic zone of the tropical Pacific Ocean (Mak A. Saito 1 ✉, Matthew R. McIlvin1, Dawn M. Moran1, Alyson E. Santoro2, Chris L. Dupont 3, Patrick A. Rafter 4, Jaclyn K. Saunders 1, Drishti Kaul3, Carl H. Lamborg5, Marian Westley6,
Frederica Valois1 and John B. Waterbury1, (Nature Geoscience volume 13, pages 355–362 (2020))
Anoxic - oxygen depleted - waters have become well known around the world owing largely to anthropogenic disruptions to the natural nitrogen and phosphorous cycles. For example, the Mississippi River Delta ecosystem - a sort of personal bête noire with me - has been destroyed by phosphorous and fixed nitrogen run off from the farms in Iowa and elsewhere in the American Midwest dedicated to making, among other things, "renewable" corn ethanol for our car CULTure. This region once was a rich zone important for its shellfish industry. Now it is dead.
The means by which this oxygen depletion takes place is called eutrophy; it involves enriching waters with fertilizers, chiefly phosphates and nitrogen in various fixed forms, leading to an explosion in the growth of algae and other microorganisms. A thick layer of these organisms takes place resulting in the depletion of the lower layers of this biomass, which then dies and sinks, whereupon oxygen consuming bacteria begin to oxidize it at a rate which depletes the waters of oxygen supply, killing all oxygen dependent life forms, fish, crabs, clams, oysters, what have you, leaving behind evil smelling messes.
These effects are known in both fresh and marine waters; briefly a few years back, these types of effects shut down the water supply to the city of Toledo, Ohio, when a concentration of toxin produced by a blue green algae stimulated by eutrophy in Lake Erie rose above safe levels.
Disruptions to the nitrogen cycle also have effects in the planetary atmosphere, resulting in the accumulation of nitrous oxide - laughing gas - a powerful greenhouse gas and ozone depleting agent.
While these effects are well known in coastal waters in various incarnations, they are less well studied in the open ocean. This paper reports on these effects in the mesopelagic zones of the Pacific Ocean - those zones between 200 meters and 1000 meters in depth.
Nitrogen oxides are well known pollutants in air; in water they are chiefly present as nitrite and nitrate; the reduced forms of nitrogen pollution in water include ammonia based species, chiefly ammonia itself, but also in some cases nitrosoamines - carcinogens that have turned up as synthetic artififacts in some medications, but are also present in foods, particularly barbecued meats and certain preserved meats.
Along with the paper's abstract, which is open sourced, and can be read by clicking on the link above, the introductory the text of the paper gives a nice overview of nitrogen biochemistry and briefly refers to anthropogenic dead zones as well:
While deoxygenation in coastal waters results in anoxic dead zones and macrofauna mortality9, less is known about the potential consequences of deoxygenation and suboxia expansion for microbial and biogeochemical processes in the open ocean. Trends for basin-scale ocean deoxygenation have been observed10,11, while modelling studies vary in their predictive capability12,13,14. Within the upper mesopelagic environment, key microbial remineralization and nutrient recycling reactions occur with the degradation of sinking particulate organic material. With nitrogen-rich proteinaceous material contributing roughly half of the biomass of microbial life, the degradation of proteins to smaller constituents, including peptides, amino acids, urea, ammonia, nitrite and nitrate, is a major mesopelagic activity along with the respiration of reduced carbon15. In this study we present global and targeted metaproteomic evidence for the preponderance of nitrite oxidoreductase (Nxr), the metalloenzyme involved in the oxidation of nitrite, within OMZ extremities of the Central Pacific Ocean, and discuss the biogeochemical implications.
The respiration of reduced carbon, of course, releases carbon dioxide.
The work herein refers to data from two expeditions, "Metzyme" in October, 2011 and "ProteOMZ" in January and February of 2016. As the names of the expeditions imply, these were efforts to identify the "proteome of the ocean."
Here is a lecture you can watch that I had the privilege of attending a few years back on the subject of mapping the genome of the ocean:
Prof. Kay Bidle, Rutgers University: The Invisible World of Marine Microbes: How Earth’s Smallest Living Things Have the Biggest Impact on How Our Ocean Works
In some ways, the proteome is more interesting than the genome, in my opinion, since the latter is an expression of what could happen or what might be, whereas the proteome (which is far more complex) is a measure of what is happening. Genes only have meaning when they are expressed. This is true in human tissue (for good and for bad), bat tissue, the tissue of roses.
The distinction between "could" and "is" is often trampled and confused as signature bad thinking. For example, I often write here on climate change, which is happening. By leaving the science forum and heading over to the curiously named "Energy and Environment" forum, you can read lots and lots of titles about what people allege could happen with so called "renewable energy," things with illiterate titles celebrating trivialities as if they mattered like for example "Texas could add 3.5 GW of solar this year." Italics are mine.
That people take this mindless horseshit seriously - typically confusing, along with "could" and "is," peak power and energy - is a good reason why we have hit 416.83 ppm of the dangerous fossil fuel waste carbon dioxide in the planetary atmosphere as of last week.
Excuse the aside...anyway...
The techniques utilized here were very sophisticated and were mass spec based using Thermo Fusion Orbitrap mass spectrometers set to high resolution.
In the earlier expedition the settings were as follows:
In the second expedition the settings were slightly different:
scans in the Orbitrap. MS2 scans had a 1.6-m/z isolation window at normal scan
rate, 50-ms maximum injection time, collision-induced dissociation activation and
a 5-s dynamic exclusion in the ion trap.
The data files processed using the mass spec manufacturer's software and then submitted to BCO-DMO and the Ocean Protein Portal data base.
The proteins of interest in this paper are known as "Nxr" proteins, in two forms, alpha and beta. "Nxr" refers to "nitrite oxidoreductase." The term "oxidoreductase" refers to the fact that these enzymes can perform either the oxidation of nitrite to nitrate, or alternatively, and perhaps of significant environmental consequence, the reduction of nitrite to N2O, nitrous oxide, "laughing gas" which despite the name isn't funny.
For quantitation, seven stable isotope labeled peptides as reference standards for Nxr alpha, and five for Nxr beta as identified from the data. These 15N labeled peptides were obtained after expression in E. Coli in a modification of a technique known as QconCAT.
The sequences of the chosen peptides are given in the Supplementary data; decent precision was obtained, except in one case, possibly because the sequence contained a tryptophan, which is sometimes subject to oxidation.
This graphic from the paper refers to the regions of interest in the expeditions:
Fig. 1: Station locations, hydrographic features and Nxr distributions for the Metzyme and ProteOMZ expeditions.

The caption:
The authors found that the NXR proteins were the most prominently expressed proteins in their analysis:
Fig. 2: Abundant Nxr in the Central Pacific Ocean and in a Nitrospira culture.

The caption:
Absolute quantitation using heavy stable isotope labeled peptides:
Fig. 3: Targeted metaproteomic analyses of peptides from Nxr at Metzyme station 3.
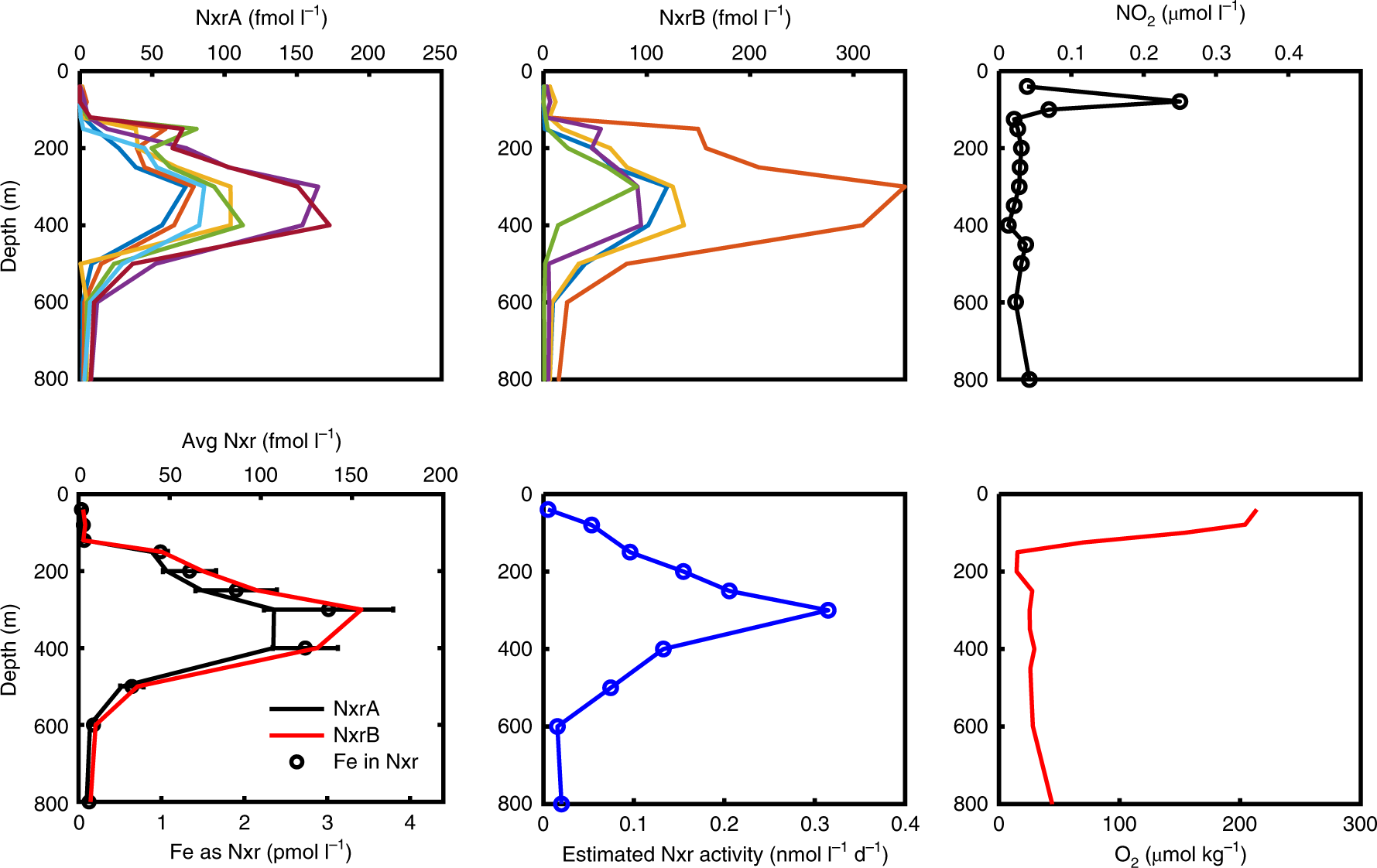
The Nxr proteins are metalloproteins, coordinating iron, which is a key point of this study.
The next graphic refers to that fact, since the open ocean is iron depleted which has a big effect on the biology of the region. There has been talk of changing this fact by seeding the ocean, on which the authors remark in their conclusion (see below).
Fig. 4: Nxr enzyme concentrations, iron use and estimated reaction rates based on targeted metaproteomic analyses.

The caption:
And now we touch on that problematic nitrogen oxide, N2O, nitrous oxide, laughing gas that isn't funny:
Fig. 5: Nxr, oxygen and nitrous oxide in the Central Pacific OMZ.
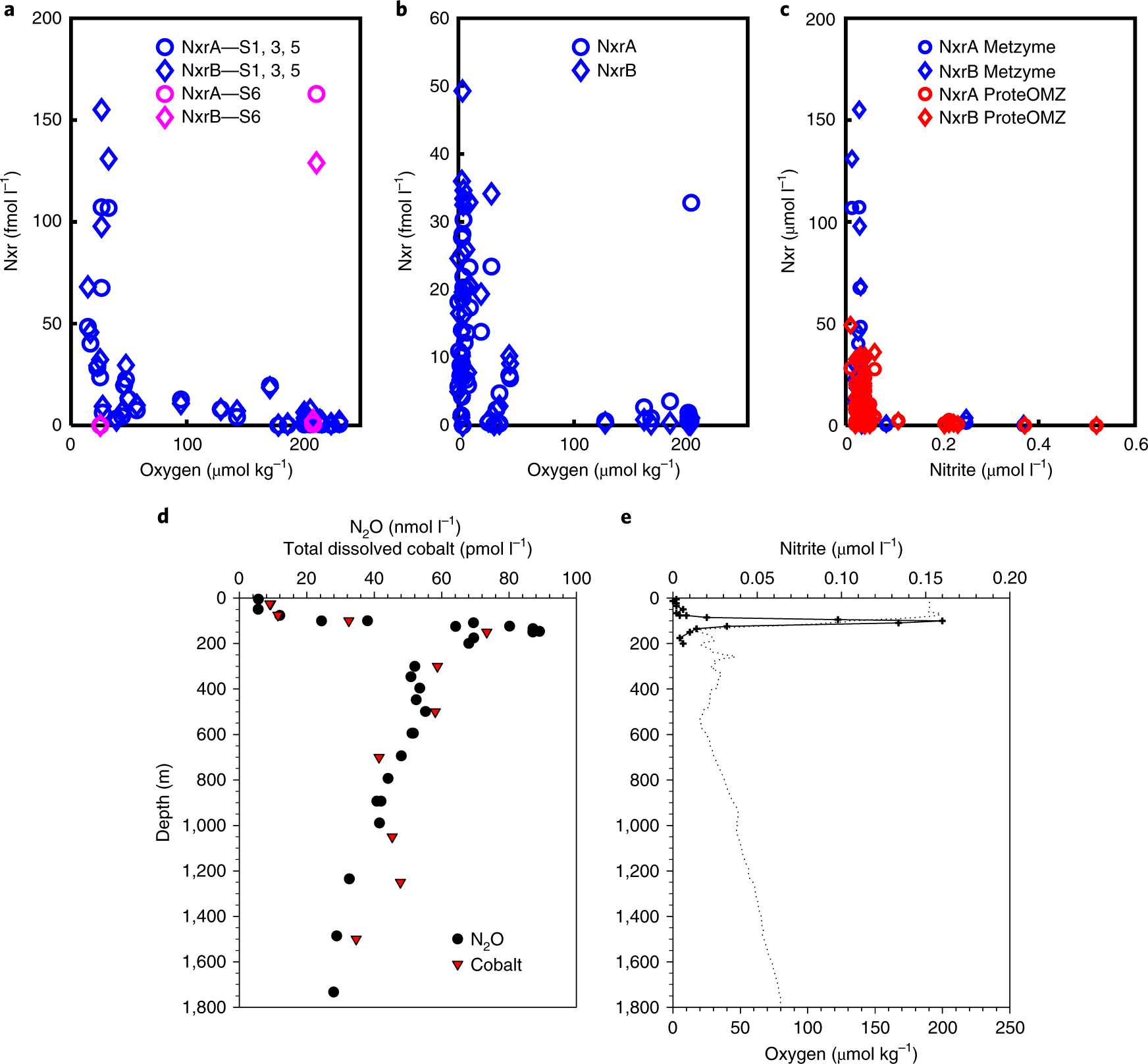
The caption:
The authors write on the issue of N2O:
This discovery of abundant Nxr in mesopelagic waters offers several new perspectives on these understudied environments. First, while the importance of trace metal nutrition to mesopelagic microbial communities has been explored for copper4,51,52, these results imply that expansion of suboxic regions by deoxygenation could result in an increased microbial mesopelagic iron demand. Iron is depleted in the euphotic zone of the equatorial and South Pacific. An inability to complete Nxr synthesis could become a constraining factor at the interface with mesopelagic depths when iron is scarce. Some phytoplankton also utilize nitrite53, but this also requires the iron metalloenzyme ferredoxin–nitrite reductase. The dependence of both nitrite utilization and oxidation on iron availability could explain the prevalence of the extensive nitrite maximum in the South Pacific (Fig. 1f,g)37. These findings could have implications for possible unintended consequences of ocean iron fertilization carbon sequestration efforts54, which may increase carbon, nitrogen and iron fluxes to the mesopelagic, reducing oxygen, probably increasing Nxr abundances and possibly increasing the greenhouse gas N2O. N2O is present in these Central North Pacific OMZ extremities below the oxygen clines and nitrite maximum (Fig. 5d,e), and ammonia-oxidizing archaea55 and NOB39 were recently shown to produce N2O, albeit through unknown mechanisms. Any eventual impacts are difficult to predict, as the myriad of interrelated processes that control the mesopelagic ecosystem and their dynamic nature are only beginning to be understood.
Reference 54 is a rather old one, designed to discredit the idea of iron seeding of the ocean to address climate change, which, as the authors note, may prove to be self defeating owing to nitrous oxide, as the authors have noted.
This is reference 54: Dis-Crediting Ocean Fertilization. (Policy Forum: Sallie W. Chisholm*, Paul G. Falkowski, John J. Cullen, Science 12 Oct 2001: Vol. 294, Issue 5541, pp. 309-310)
I recall discussions of this iron seeding scheme either here when I was writing in the Energy and Environment forum before I began to avail myself of the marvelous "ignore" button here, or over at Daily Kos, where I was ultimately banned for telling the truth.
Apparently some people believed that entirely screwing up another biome, in this permutation, the open ocean was preferable to the use of nuclear energy to displace all fossil fuels, nuclear energy being the only sustainable form of energy available to humanity.
Go figure...
It is important to note that the situation with respect to fixed nitrogen is not merely a function of anoxia in bodies of water. It is very much connected, on land as well as at sea, with synthetic ammonia based fertilizers, without which we would simply be unable to feed more than seven billion people on this planet. The problem is intractable, and we should start paying attention, but we won't, as we have a long record of paying lip service to environmental issues and offering bizarre ineffective remedies which are not serious and should not be taken as such.
Right now the world's attention is focused on a viral particle, an issue that will prove ultimately to be ephemeral.
Climate change will still be here when we're done worrying about the virus. It is getting worse, not better, rapidly.
Despite the strain and stain of the times, enjoy waking up tomorrow. The beauty of the world is awesome, and we ought to appreciate every breath, something I hope we realize even as we realize our lives are fragile.
I_UndergroundPanther
(12,480 posts)When the hydrogen sulphide starts blowing inland from all those rotting dead zones.
http://jumpingjackflashhypothesis.blogspot.com/2020/05/event-update-for-2020-05-10.html?m=1
NNadir
(33,538 posts)...in connection with sargassum, which has been washing up on South Atlantic and Caribbean beaches in ever larger amounts, probably as a result of eutrophication.
Its rotting has not, to my knowledge, resulting fatal levels of hydrogen sulfide, but it is unpleasant.
I wrote about Sargassum in this space: Can We Recover Carbon Dioxide From the Atmosphere Using Sargassum Seaweed? and The Very Modest Carbon Capture Potential of the Massive Sargassum Blooms.
In the open ocean, I wouldn't worry too much about it though, since hydrogen sulfide tends to precipitate in the presence of transition metal ions, including but hardly limited to iron. (Many ores for metals are in fact, sulfides, zinc blende, cinnabar, etc, which is why reducing the metals via smelting represents such an environmental problem.)